Molecules on Surfaces and Catalysis
 |
Molecules bond to surfaces because they interact with the surface atoms. These interactions may be weak, of the 'non-bonding' type with the molecules themselves staying intact when they stick to the surface (or are adsorbed). Alternatively, they may involve the formation of new chemical bonds between the surface atoms of the adsorbed molecule, which necessarily involves extensive reorganisation of the bonding within the molecule; in simple molecules such as hydrogen (H2) and nitrogen (N2), dissociation into component atoms on the surface will often follow - a point to which we return below. This reactive type of interaction is generally known as chemisorption in contrast to physisorption the term used to describe the weaker unreactive binding of molecules to surfaces. Among the many fascinating questions that we can pose about the problem of adsorption, the first relates to the structures and energies of the sorbed molecules; the second to the kinds of chemical reactions which can occur within and between molecules that have been captured on surfaces. The third and perhaps most subtle questions concern the way in which the properties of a material are modified by the sorption of molecules on its surface.
During recent years, a whole range of highly sophisticated experimental techniques have been developed for probing the properties of molecules on surfaces. We have already referred to the new surface microscopes. Many spectroscopic methods have been tailored to the study of surface species as have diffraction techniques. Once again, however, the computer is emerging as an exceptionally powerful tool in this field. The images on this page show examples of computational models for these topical and important systems.
The image below illustrates how a diphosphonate molecule[C3H6(PO4)2] can bind into the surface of barium sulfate BaSO4. The mode of interaction is particularly interesting: the PO4 groups of the diphosphate replace the SO4 groups on the surface, enabling the molecule to lock in very effectively onto the surface of the material. Interestingly, the molecule acts as an inhibitor for the growth of BaSO4 - a highly insoluble material that aggregates and can eventually block oil pipes - a major problem in the oil industry. Inhibitors like the diphosphate coat the surface of the crystal, thereby preventing growth, are of great practical value. The computer calculations, as illustrated here takes us to the heart of the surface chemistry of this industrially important process.
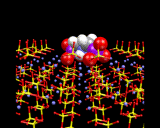 |
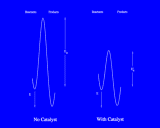
Once molecules have been sorbed on surfaces, they can undergo new types of chemical reaction; or reactions which can take place with difficulty in a gas or liquid may occur readily on surfaces. This may be because the molecules are brought into close proximity; or it may be a consequence of changes in bonding consequent upon chemisorption. In either case, surfaces have the crucially important property of catalysing chemical reactions. Catalysis is, of course, one of the key processes in chemistry and biochemistry. Catalysts are agents which facilitate chemical reactions without being either a reactant (the starting point of the chemical reaction) or a product (the end point).The way in which they operate is illustrated in a general way on the left.
Recall that chemical reactions, other than those which take place immediately and spontaneously, are opposed by an 'energy barrier' which the system must surmount on the path from reactants to products; this barrier is often associated with the breaking of bonds in the reacting molecules prior to the formation of new bonds in the products; and the height of the barrier is usually the major factor controlling the ratio of a chemical reaction: high barriers result in slow reactions. Catalysts work by reducing the height of these barriers. They may, for example, help bond-breaking in the reactants; a different, but largely equivalent, description is that they stabilize the 'intermediates' involved in the reaction, that is the high energy species corresponding to the maximum in the energy barrier (which may correspond to some well defined, if unstable, chemical species with a short but definite life time). The surfaces of metals and of oxides promote a rich and varied range of catalytic processes whose economic importance is difficult to overestimate as they are the basis of many key processes in the chemical industry and are responsible for billions of dollars of products worldwide each year. These heterogeneous catalysts, so called because the catalysis takes place at the interface between two phases - gas and solid - should be contrasted with homogeneous catalysis which takes place entirely within a single phase (gas or liquid). Homogeneous catalysts are also of considerable industrial importance; moreover, this type of catalysis is essential for continued functioning of living systems.
 |
 |
Bond dissociation is one of the simplest but most effective ways in which surfaces can catalyse reactions. We will consider just two of many examples. Hydrogenation (which consists of adding hydrogen atoms to the molecule) is a common and important industrial reaction. Many such reactions involve adding H atoms to double bonds in unsaturated molecules. However, there is a strong bond between the two atoms and the hydrogen molecule and so if a hydrogen molecule and, for example, an unsaturated molecule simply bump into each other, there will almost always be insufficient energy in the collision to allow the bond in the hydrogen molecule to break. However, if the molecule is adsorbed on to the surface of a molecule such as platinum, the hydrogen molecule breaks in to its two atoms (dissociates) because each hydrogen atom makes a new bond with the surface metal atoms. These surface hydrogen atoms can now diffuse over the surface and attack unsaturated molecules as shown schematically on the left. Perhaps an even simpler example is provided by the deceptively simple reaction between hydrogen and oxygen:
2H2 + O2 = 2H2O
Gaseous hydrogen and oxygen can exist together in the same gas jar without reacting because again the H and O atoms are strongly bound within their component molecules, and although they collide with each other billions of times a second, again there is not nearly enough energy in the collisions(certainly at ambient temperatures) to break the bonds. However, if we add just a few grains of metallic platinum to our jar, the mixture will explode, as both the hydrogen and oxygen molecules can dissociate on the surface of the platinum and then react with each other.
A more topical example is provided by 'de NOx' catalysis. Nitrogen oxides (principally nitric oxide, NO) are a serious pollutant, being acid and corrosive gases produced by combustion in car engines and in power stations. There is currently major environmental and economic pressure to provide cheap and efficient ways of removing these gases from car exhausts and power station effluents. Certain metals, e.g. palladium and platinum, can catalyse the decomposition of these oxides, and the mechanism involved has been revealed by recent computational calculations as illustrated. The challenge is now to devise cheaper, low temperature and more effective catalysts for this crucial reaction.
As a final example (of the many we could have chosen) of heterogeneous catalysis, we take one of the most famous and important reactions in the development of the chemical industry, the Ziegler-Natta polymerisation catalyst - one of the first commercial routes to low cost polymers, whose production by the chemical industry in this century has so markedly influenced our lives. This catalyst, which converts ethene to polyethylene comprises an ionic compound, magnesium chloride (MgCl2) to which we add titanium chloride and related molecules. These molecules create the active sites on the catalyst. The concept of the active site is a crucial one in the theory of catalysis. Often only a small proportion of sites on a surface can effect the catalytic reaction; they may be associated with the kind of surface irregularities (steps, kinks and defects) discussed earlier; or, as in this case, different types of chemical species may create the sites. The type of active site thought to be involved in the Ziegler-Natta catalyst is shown below. The stability of such sites, which comprise the titanium bonded to carbon as well as chlorine, has been suggested recently by computational studies.
 |
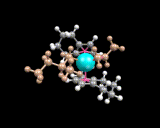 |
Ethylene polymerisation can also be effected by 'homogeneous' catalysts, which are usually complex organometallic compounds of the type shown on the left. Again the mechanism involves the ethene (or other unsaturated) molecule being inserted into the metal-carbon bond.
Perhaps the most fascinating class of heterogeneous catalysts are the zeolites. As we saw, these materials are all surface, and both the acid sites in their frameworks and the metal ions in the pores are responsible for a huge variety of catalytic processes. Moreover, the geometries - pore and void dimensions - of the zeolite structures strongly influence the selectivity of the catalysis - that is the ability of the catalytic process to produce a specific product in high yield rather than a range of products. Increasing selectivity as well as better yields at lower temperatures are among the strongest economic pressures in the field of catalysis. Enzymes, which facilitate the chemical reactions necessary for life, are the envy of the catalytic chemist.
 |