Microporous Solids - Cages, Channels, and Voids
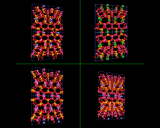 |
The feldspar structures of the previous section, and shown here, are made by nature's crucibles - volcanoes which furnish the fiery high temperature conditions which favor the formation of these dense "igneous" rocks.
Nature is, however, a wonderfully varied synthetic chemist, and has learned to make other subtler structures by using gentler conditions. In the deserts of Nevada in the United States of America huge deposits are found of a low density aluminosilicate known as phillipsite. These minerals were synthesized millions of years ago when this land lay under water. High concentrations of silicon and aluminium in the water at the bottom of this ancient ocean slowly crystallized out with SiO4 and AlO4 tetrahedra linking together to form not a compact, dense but an open structure which is porous at the atomic level - a microporous structure.
 |
These natural synthetic conditions may be reproduced, refined and varied in the laboratory. And there are now a wide range of both synthetic and naturally occurring microporous aluminosilicates, known as zeolites. Some of the simplest and most important structures are built from the high symmetry cubo-octahedral cage which may be described as a truncated octahedron, with a ring of four silicon or aluminium atoms (linked by oxygens) replacing each of the corners and a ring of six Si or Al making each of the faces.
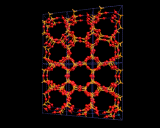
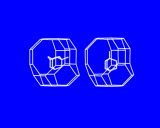
These cages may themselves be fused across the four rings as shown to generate the structure of the natural and synthetic mineral sodalite. An alternative and cleverer principle used by these structures is to bridge across the four rings. In fused structures the four rings are shared between neighboring cages; in bridged structures, four rings in one cage are linked to similar rings in a neighboring cage by oxygen atoms which connect the tetrahedral atoms (Si or Al) in the two rings. The resulting structure shown is known as Zeolite A and is widely used in detergents. Bridging across six rings generates another structure - Zeolite Y illustrated which is used on an enormous scale in the petrochemicals industry as a "cracking catalyst". Long chain molecules of the type present in heavy oils may diffuse through the pores of this structure and into the large cavity shown in where they may be broken down into shorter chain molecules - of the right length for petrol/gasoline.
 |
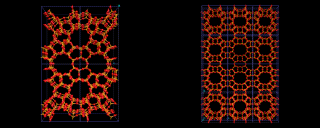 |
Other structures are illustrated on the left. Zeolite ZSM-5, shown contrasts with Zeolite A and Y discussed above in that it is built from 5 and 10 rings.
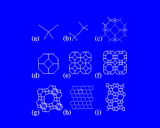 |
A fascinating variety of channel structures is also found. This ZSM-5 has interpenetrating straight and wavy (sinusoidal) channels; mordenite has a purely straight channel structure. Indeed in these microporous structures nature seems to be exploring as widely as possible the topological possibilities of corner shared tetrahedral networks.
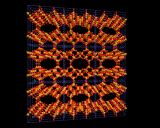
Since zeolites are aluminosilicates, they normally contain metallic ions that neutralize the negative charge of the aluminosilicate framework; these commonly occupy well defined sites close to the rings in the cage structures. The image on the left highlights the positions occupied by cations in the Zeolite Y structure; especially interesting is the prism position located between the bridged six rings in the structure which provides a low energy site for many metal ions.
It is important to realize, however, that these structures are only seen in 'dehydrated' materials in which excess water which is always present in the structures after synthesis, has been driven off (usually by heating). The water molecules present in 'hydrated' systems stick to and surround the metal ions which then occupy ill-defined sites in the voids and channels. These loosely bound hydrated metal ions can easily be taken out of the structure and exchanged for other metal ions. And 'ion exchange' is indeed one of the oldest applications of these minerals. For example, Zeolite A is effective at exchanging hydrated sodium ions in its pores with calcium and magnesium ions in surrounding water - the ions that are responsible for the 'hardness' of water as they form precipitates (insoluble substances) with the species present in soaps and detergents producing the unsightly scum familiar to those (like the residents of London) whose water supply is hard. The ion exchange process effected by Zeolite A (and other zeolites) 'soften' the water and zeolites are now widely used in detergents as water softeners; they are more environmentally friendly than the alternative phosphate systems which act as nutrients for microrganisms thereby disturbing the ecological balance of rivers and lakes into which detergent wastes are discharged. A more spectacular and 'high tech' application of the high ion exchange capacity of zeolites was in the use of the naturally occurring clinoptilolile in removing radioactive metals from contaminated water after the Three Mile Island Nuclear Accident in 1982. This zeolite was able to extract completely a wide and diverse range of metallic ions from this dangerously contaminated effluent.
 |
 |
A simpler alternative to neutralisation of the negative charge of the framework by cations is to incorporate protons (or H+ ions), which stick to the bridging oxygens and provide highly acidic groups. Zeolites may therefore behave as exceptionally strong solid acids which allows them to initiate a range of reactions in molecules present in their pores. The type of reaction will, however, be influenced to a pronounced extent by the pore architecture, which controls the shape of the molecules that can enter and leave the pores, and the type of reaction which can take place within them. Zeolites, owing to their acidity, are therefore potent catalysts; but the catalysis is shape selective; it is controlled by the pore and void structures of the zeolite. These unique catalytic properties are widely exploited in industry. We have already mentioned the process of catalytic cracking effected by zeolites in which acid centers attack long chain hydrocarbon molecules, which are broken down into shorter chain species. The reverse process we saw was effected by ZSM-5 which acts as a hydrocarbon synthesis catalyst. Other reactions such as isomerisation (changing shape) and alkylation (adding of carbon atoms) are also of major industrial importance. Indeed zeolites are to the inorganic world what enzymes are to molecular biology.
 |
The microporous architecture of these materials results in a third range of applications in gas separations. Different shaped molecules are sorbed into and diffuse through these structures to different extents. Zeolites may therefore be used to separate mixtures of different organic molecules and even the nitrogen and oxygen molecules present in our air.
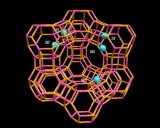
Detailed understanding of both catalysis and sorption require knowledge of the way in which molecules fit into the pores of these zeolites. Computational methods are now providing extraordinary insight into these processes.
 |
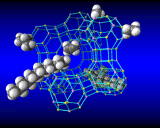
Computational methods also beginning to provide insights into one of the greatest mysteries of zeolite science - the mechanism of synthesis. It has long been known that to synthesize certain pore architectures, it is necessary to introduce organic bases (nitrogen containing organic molecules) to the synthesis gel. Computational methods are now showing how these species fit inside and stabilize the zeolite structure as shown.
 |
Microporous science has continued to diversify with the extension of the class to include aluminophosphates (built up of AlO4 and PO4 tetrahedra), which allow new pore architectures, like the exceptionally wide pore material 'VPI-5' discovered by Mark Davis at Virginia Polytechnic. And considerable excitement has been generated recently by the discovery of silica systems with exceptionally wide pores (so-called mesoporous materials) whose architecture is also illustrated. There is no doubt that the science of this extraordinary range of materials still holds many surprises.
The microporous materials represent probably the most sophisticated and complex of crystalline (that is, ordered) inorganic structures. Their applications in catalysis and gas separation also illustrate the interface behind the inorganic and organic worlds which is of such importance in contemporary chemistry.
 |
 |