Rings and Chains
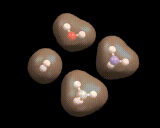 |
Some atomic assemblies are simple. As we have seen, hydrogen atoms stick to each other to form the H2 molecule. Hydrogen atoms stick to oxygen atoms to form the water (H2O) molecule, to nitrogen to form ammonia (NH3) and to carbon to form methane (CH4) - as shown on the left. Oxygen binds to carbon to form carbon monoxide (CO) and carbon dioxide (CO2), also shown on the left). These and many other compounds based on small molecules are immensely important; for example, water makes up 80% of our body weight and methane is a widely used fuel (being the main constituent of natural gas). CO2 has been implicated in global warming - this small molecule, produced in vast quantities, could change the climate of the world.
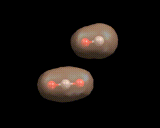 |
The architectural possibilities in small molecules are, however, necessarily limited. To achieve complex structures, there are a number of simple games we can play with atoms. The first is to form chains. And the element which uses this trick most widely is carbon. The simplest carbon chain compounds are the paraffins - chains of carbon atoms with hydrogens bonded to the carbon shown on the left.
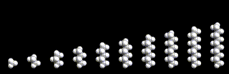 |
The chain may be straight or branched as seen in the figure. Moreover, there are no restrictions on their length, and the properties of the resulting compounds vary with length. Paraffins containing 2, 3, or 4 molecules are gases under normal conditions (and are widely used as fuels). From 5 to 8 carbon atoms, we have light fluids. (Petrol/gasoline comprises molecules of this size.) As the chain length increases, the liquid becomes more viscous and for longer chains (greater than approximately 20 carbon atoms)waxes and tars are formed. These simple and straightforward chain compounds, whose structures are perhaps slightly dull, are nevertheless of great importance in modern society. The molecules are rich in energy; and the energy can be released when they react with oxygen present in air, which is, of course, the basis of their widespread use as fuels.
 |
Chain molecules are also widely produced by the modern chemical industry. Indeed the plastics and fibers which are so widely used today are polymers. Such chains contain a basic unit - a small group of atoms which is repeated tens, hundreds and thousands of times. Again the properties of the resulting materials depend upon the chain length.Typical and important polymers are illustrated on the below.
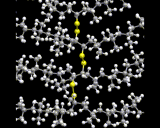 |
Polymer chain molecules, of course, pack together in liquids and solids - a topic to which we will return later. An intriguing form of chemical interaction between polymers is 'cross-linking' where atoms from one polymer chain form chemical bonds with those in neighboring chains; a typical example is shown here.
Such processes may dramatically alter the physical and chemical properties of the polymer compound.
By far the most sophisticated, intriguing and important chain molecules are those present in living matter. Indeed, living matter has perfected the art of designing chain molecules for precise and particular chemical and physical tasks.
 |
Moreover, we again find that living matter uses rings of atoms. For example, sugar molecules - one of the main fuels of animals and plants - are built from rings of carbon and oxygen atoms as illustrated.
 |
The next strategy in achieving complex structures is to extend into two and three dimensions. Such structures will be considered in Atomic Scale Architecture. Meanwhile, having gained some understanding of molecular architecture, we consider the next obvious question, that is, how molecules interact with each other.