Molecular Shape
 |
We know, from the previous discussion, what makes atoms stick together, but what limits the proportions in which they can combine and what factors control molecular shape? The simplest and most basic factor arises from the relative sizes of the different atoms. The concept of atomic size is, however, somewhat fuzzy: the size of an atom is controlled by the extent of its electron charge cloud, and the density of electrons around an atom does not suddenly reduce to zero; it gradually decays. Nevertheless it has proved possible to assign approximate radii to atoms (as shown on the left). And purely geometrical factors will limit the ways in which we can pack atoms together. These factors become most obvious when we consider packing in crystals; thus in crystal structures containing, for example, the large caesium ion, there are often eight other atoms surrounding each caesium; whereas the smaller lithium ion has room for only four. We return to these considerations when we discuss the structure of crystals.
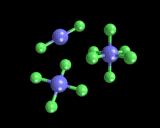 |
The next point is that atoms have well defined combining powers (the chemical concept of valence) which arises from specific features of their electronic structure; in particular the number of electrons in the outermost shell relative to the total number of electrons that can be present in that shell. Indeed, the concept of valence springs from one of the oldest and most powerful ideas of theoretical chemistry - the 'electron pair' bond. Chemical bonds (like those between the two hydrogen atoms in H2) often have a pair of associated electrons. And when there are different numbers (four or six) we often refer to them as 'double' or 'triple' bonds (for example, an oxygen molecule has a double and a nitrogen molecule a triple bond). Chemical bonding can be thought of in terms of the drive by atoms to achieve stable configurations in which all their outermost orbitals are full, which can be effected by teaming up with other atoms with which they 'share' electrons in pair bonds. Thus the atom carbon has 4 electrons in its outermost shell which has 'room' for a total of 8; so carbon has a valence of 4 (as in the compound methane, CH4). Oxygen has 6 electrons and again room for a total of 8; hence it has a valence of 2 as in water, H2O. The value of this approach is shown by the fact that atoms in which the outermost shells of electrons are full are chemically inactive. These are the famous "rare gases" (many of which were isolated by Sir William Ramsay working at University College London at the beginning of the twentieth century). Thus helium and neon have no chemistry; the atoms bond neither with themselves nor with any other species. Argon and krypton show very limited chemistry because it turns out that the outermost shell of electrons can, with difficulty, be expanded. In one of the remarkable developments of chemistry in the 1960s, it was found by Neil Bartlett and coworkers that xenon has a quite extensive chemistry as it is relatively easy to expand its outermost shell. Some of the compounds xenon forms are shown on the left.
The more traditional chemical concepts of valence and pair bonds are fully compatible with the approach to chemical bonding in terms of electron density redistribution which we presented earlier. The former allows us to understand why atoms form bonds; the latter guides us as to the constraints on the molecular structures that can form. A key feature of chemical bonding to which we now turn concerns the fact that it can have specific directional requirements. Thus the carbon atom in the majority of its compounds bonds to four atoms which are close to the corners of a tetrahedron; although in a significant proportion of cases it adopts a rather different bonding pattern with three surrounding atoms close to the corners of an equilateral triangle and in some gases a linear arrangement is adopted. These specific geometrical requirements follow naturally from the criteria that the resulting assembly of atoms should have the lowest possible energy. More specifically, they can be understood in terms of the different shapes of the atomic electron density charge clouds and by the ways in which these can interact. Chemical bonding occurs where the atomic charge clouds interact and overlap with each other.
 |
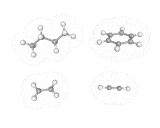 |
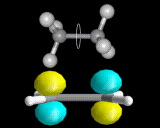
Just as the differently shaped atomic charge clouds are labelled s, p and d, so the different shaped molecular charge clouds (often referred to as molecular orbitals) are labelled σ, π and δ. Examples of molecules containing these different types of bonds are shown above. These different types of bond have different properties, sigma bonds concentrate electron density directly between the nuclei where the interactions between electron and nuclei are strongest, in π bonds the maximum in the density is away from the internuclear axis.
Sigma bonds are often more stable and π bonded molecules more reactive because the bonding electrons can be reached more easily in the case of π electrons. There are subtle geometrical differences as well. We can rotate about σ bonds but, as shown below, rotation about a π bond destroys the orbital overlap and is energetically unfavorable. π bonded molecules like C2H4 are therefore more rigid than σ bonded frameworks.
 |