Inserting Atoms Between Tetrahedra
 |
The minerals from which our planet is constructed are made mainly of silicon, oxygen and a variety of other atoms of metallic and non-metallic elements. These structures are mainly made from SiO4 tetrahedra, which as we saw earlier, may link together by sharing corners. In silicon dioxide, all corners are shared. The large number of different structures known for this compound indicate the diversity of the topology of corner shared tetrahedral networks.
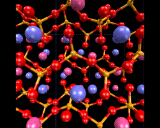
In silicates, extra metal and oxygen atoms are inserted. The additional oxygen disrupts the three dimensional networks; it can only be accommodated by creating 'non-bridging' oxygen species - 'unshared' corners of the SiO4 tetrahedra, as illustrated. The type and 'dimensionality' of the resulting network depends on the number of these non-bridging oxygen, or conversely (or perhaps more appropriately) on the number of corners that remain shared. Thus if three corners are shared, the tetrahedra will link together to form sheets.
The layers of atoms in the resulting crystal structures can often easily slip over each other. And it is not surprising that many minerals which adopt these structures - in particular the clay minerals - are soft materials. As discussed further below, clays normally contain aluminium as well as silicon, and a classic example is kaolinite, the main constituent of white clay.
 |
 |
Layer structured minerals exploit all the architectural possibilities of these triply linked corner shared networks. In some cases, the non-bridging oxygens are all on one side of thelayer; in others they alternate. The layers may be buckled and in some cases may even roll up! Further complexity arises from the different arrangements of the metal atoms (which recall enter with the excess oxygen), and from the possibility already referred to of including aluminium in the networks. We will consider both these aspects later.
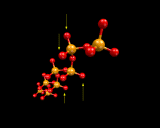
Returning, however, to the next mode of tetrahedral linkage, in which only two corners of each SiO4 tetrahedron are shared, we generate silicate chains.
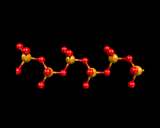 |
 |
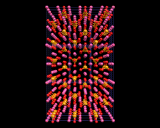
Again silicate chemistry explores the range of structural possibilities provided by catenation (chaining) of tetrahedra; and chain structures are widely found in naturally occurring minerals, for example diopside. When only one corner is shared, then only two tetrahedra may be linked. Such structures, although rare, are known and in the high pressure phase of Mg2SiO4 which we will discuss at the end of this chapter, when we consider in more detail the materials from which the earth is made.
 |
Finally, silicate tetrahedra may share no corners, and there are several structures based on isolated SiO4 tetrahedra. The most important example is probably the dense mineral forsterite, whose structure is illustrated. A variant of this compound - the mineral olivine which contains appreciable amounts of iron - forms the major component of the upper part of the earth's mantle, as will be discussed later.
 |
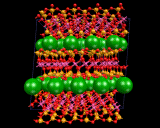
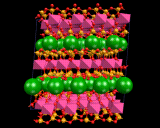
Let us now consider how we insert the metal atoms. These go to positions between the silicate layers, chains or isolated tetrahedra. The latter all have a negative charge as negatively charged oxygen ions have been inserted. The metals are positively charged ions; and the electrostatic interactions between the two binds the crystal. In the case of the layer structures, the metal ions are themselves situated in layers between the negatively charged sheets as shown And they occupy precise and well defined sites between the silicate chain in, for example, MgSiO3 and between isolated SiO4 groups in the mineral forsterite referred to above.
 |
 |
Further variety is built into silicate structural chemistry by the possibility of including aluminium as well as silicon in the structure, which occurs readily as the two types of atoms are very similar in size. In many structures, AlO4 tetrahedra may simply replace or substitute for SiO4 tetrahedra - a simple trick which, however, allows a vast extension of the range of possible compounds. But unlike silicon, aluminium is commonly stable as AlO6 octahedra, so octahedrally coordinated structures become possible in aluminosilicate chemistry. Many such examples are found in the structure of clays where the aluminosilicate layers are commonly constructed of both AlO6 octahedra and SiO4 tetrahedra.
Let us return, however, to the simple expedient used by nature of replacing silicon by aluminium in corner shared tetrahedral networks. The consequences here are far more wide ranging than we might at first imagine.The nucleus of the aluminium atom has one less proton - one less positive charge - than that of silicon. So when silicon is replaced by aluminium, the aluminosilicate network becomes less positively, that is more negatively, charged. To balance this extra negative charge, additional positively charged metallic ions must be added to the structure. The most obvious consequence of this simple principle is that fully corner shared aluminosilicate networks may be constructed which also contain positively charged metallic ions. In contrast, if we are confined to SiO4 tetrahedra, then networks in which all corners are shared necessarily have the composition SiO2; and as we have seen, a large variety of SiO2 structures are possible. In aluminosilicates this range of structures is still further extended as the forces of attraction between the negatively charged framework and the positively charged metal ions stabilise many different types of fully corner shared network.
Dense, fully corner shared aluminosilicate networks provide some of the hardest and most enduring of minerals. We have illustrated the structure of typical feldspar minerals which are present in hard rocks such as granites and basalt. At the other extreme there is a fascinating class of complex low density structures which provide perhaps the most beautiful and subtle structures known in the inorganic world.
 |