Atoms on the Move
 |
The models shown so far of atoms and molecules might lead us to think that the microscopic world comprises frozen, static structures. Reality is very different. Atoms and molecules are in constant motion and even at low temperatures, matter is very dynamic at microscopic levels. Molecules constantly rotate and vibrate; they translate (move bodily) in gases and liquids. They may also move, but very much more slowly, in solids.
Let us look at these common forms of molecular motion, where we will find once more that our understanding has been enormously assisted by the computer. Translation is the simplest. In gases, which contain low densities of molecules, molecular motions are remarkably fast. For example, a hydrogen molecule in the gas under ambient conditions is traveling on average at 6,940 km/h. Of course it is constantly bumping into other hydrogen molecules and the walls of the vessel (and it is the latter collisions that give rise to the pressure exerted by the gas on the walls of the vessel). As the temperature rises, the molecules gain more energy so at 1000 degrees Centigrade on average our hydrogen molecule will be moving at 14,343 km/h. The pressure due to the collisions of these more energetic molecules will be correspondingly higher.
 |
The higher densities of liquids mean that molecular motions are far slower. But molecules are constantly on the move in liquids; they push and jostle their way through their neighbors. Below, for example, the trajectory of a water molecule in liquid water over a period of one ten thousand millionth of a second (10-10 sec) is illustrated. We see once more how on this time scale, a lot can happen at the molecular level.
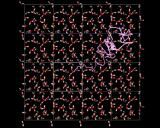
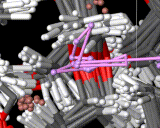
Molecules in solids are usually firmly anchored to particular sites. Diffusion in solids is therefore slow and commonly requires imperfections or defects in the solid to allow it to proceed. If, for example, an atom or molecule is missing from its regular site, a neighboring molecule can jump into this site allowing diffusion to occur. Such processes are of central importance to key solid state reactions including corrosion. There is, however, an important and exciting class of solids in which rapid atomic migration takes place within the solid phase. Indeed, in the important class of 'fast ion conductors', ions move at speeds in solids which are typical of those seen in liquids. The figure shows a computer simulation of the way in which the lithium ions diffuse in a conducting polymer. The exposure time is again 10-10 seconds during which one of the ions (colored pink in the image) moves between many different sites.
 |
Molecules in gases and liquids are also constantly rotating as they move. Oxygen (O2 and nitrogen (N 2) molecules will rotate around 1010 times per second at ambient conditions. The greater interference between the molecules will of course slow down their rotation as well as their translation in the liquids. More complex molecules show internal rotations in which only a part (or chemical group) of the molecule rotates.
Such rotations are commonly 'hindered', that is the rotation of the group is opposed, by interactions with other parts of the molecule.
Thirdly, molecules vibrate just as balls held together by springs vibrate. Thus the oxygen atom in the oxygen molecule vibrates (that is the bond shortens and lengthens) 1014 times per second. The larger the number of atoms, the more complex the nature of the vibrational motion. The frequency of a vibration depends on the forces acting on the atom, and is a constant that does not change with temperature. However, as the atoms acquire more energy at higher temperatures, their amplitudes increase, that is, the extent to which the bonds compress or expand, increases.
 |
Atoms and molecules vibrate in solids. Indeed, since translation and rotation are commonly not possible in the constrained environments in solids, this is the only type of motion that is possible. Again the amplitude of the vibrations increase with temperature. We show a computer simulation of the vibrational motion in ice close to its melting pint.
The study of atomic motions - of the dynamics of matter at the atomic level - is one of the most widely investigated fields of physical chemistry; and it is one where, as we have already seen, the computer is playing an increasingly valuable role. In the next section, we explore how, in this and in other fields of chemistry and physics, the computer is a tool of growing power and importance.