Protein Folding
 |
Why not transfer perfect copies of the genetic information? Some organisms do this and they accept unchanging roles on the earth. The combining of genetic information allows a species to adjust succeeding generations in response to a changing environment. If one particular characteristic is especially desirable, the pattern for a particular molecular machine perhaps, this can be emphasized in the molecular makeup of new generations. In 1859 Charles Darwin published The Origin of Species which set out the harsh principle which determines the direction of evolving molecular changes - the survival of the fittest. Those characteristics that yield life forms best able to cope with the circumstances of their existence will tend to survive and be solidified for the organism.
The DNA mechanism copies information extremely accurately - in fact the organism has specific molecular machines that are designed to repair any damage that may occur to the all-important molecular blueprints. How then does any variation between individuals arise? It turns out that very rarely a mutation or modification will be incorporated into the information core that is not detected and repaired. Such a mutation is often disadvantageous, and will result in an organism that cannot survive and reproduce. But sometime the resulting new characteristic will be a vital step in the continued survival of the species. For example a point mutation in the sequence of the haemoglobin molecule causes sickle cell anaemia and a frightening reduction of the efficiency of red blood cells. In the case of this mutation, people who are unfortunate enough to have inherited this coding have enhanced resistance to malaria infection may account for its widespread distribution. Nature makes random rare explorations of the possibilities of code modification with survival of the fittest in the existing environment determining whether the pathways explored are retained or discarded. This principle has allowed molecules and the information demanded for the construction of organisms to evolve for much of the time that the earth has existed.
Indeed, four thousand five hundred million years ago there was no life on earth, there was no atmospheric oxygen and the temperature at the surface of the planet was considerably higher than it is today. However, although this environment might seem to us today to be particularly inhospitable (almost as alien to us today as the surface of another planet) it was not only chemically rich but also prepared for change. Ultraviolet radiation from the sun, unchecked by an ozone layer, electrical discharges in the form of lightning, and constant geothermal heating provided the stimulus necessary to provoke the formation of a constant and diverse range of organic molecules. Eventually molecules were formed which were capable in a primitive way of producing copies of themselves. Perhaps these first reproducing molecules were seeded by the carbon containing molecules formed on and transported by meteors. Alternatively the regular layers of crystalline minerals, such as the clays, may have served as the original templates and catalysts of molecular reproduction. The details of the first reproducing molecules are inevitably obscure and we will probably never be certain of the details of their existence. We know that the environment was chemically harsh, that evolutionary pressures were therefore strong, and the time scales involved can be now measured only in geological terms, and we can only speculate as to which molecule led the way in this molecular revolution. The first reproducing molecules, perhaps too small to leave detectable fossil evidence, were probably consumed as the constituents of later evolving molecular systems. Just as we today happily exploit the residues of our very distant ancestors in, for example, the form of oxygen released by the use of carbon dioxide as a feedstock by early cellular systems and oil made from their fossilized remains.
The search for the first reproducing molecules can be compared with an attempt to extrapolate backward from a modern computer across just a few decades to the first electromagnetic digital code breakers used in the Second World War and back further still to the card controlled weaving machines of the nineteenth century. If the only evidence to hand is the present manifestation of the evolution, today's computers, the determination of distant ancestors is clearly difficult. An alternative approach is to produce a model that attempts to encompass the conditions under which the evolution took place. Needless to say if the process took billions of years this may be a lengthy simulation but with the audacity of those confined to human time scales, scientists have, on occasion, set out to mimic the conditions which are believed to have existed at the dawn of our world. Experimentally it has been demonstrated that carbon dioxide, H2O and the simplest molecules can be combined under suitably primordial conditions to yield amino acids, essential building blocks of proteins. We have examined the reasons behind chemical bonding in the previous chapter. Once formed how might simple molecules arrange to produce copies of themselves? Replicating systems of molecules may have arisen on earth on more than one occasion only to die out as the environment dramatically changed for the worse as the result of a meteor impact or dramatic geological upheaval. As noted above, one appealing theory couples the tendency of solids to form repeating arrays of atoms capable of promoting chemical reactions with the need for the first simple molecules susceptibility to organization. According to this hypothesis the solids and organic molecules formed an early sympathetic relationship, the solid phase providing the regular catalytic and templating functions and the smaller molecule in turn holding the solid phase together prior to its own formation of a chain like structure. The clay deposits seen on the earth today might be the remnants of an extremely early chemical form of molecular life. Whatever the earliest molecular steps toward life were, we can see the descendents of the surviving successful chemical copying mechanisms in the forms of life on earth today.
Humans are a comparatively recent region of explorations for the molecules of life. Interestingly humans, who have the appropriate coding to be marvelous mimics, have achieved much through understanding natural processes and then imitating them. In mathematics, for example. so called genetic algorithms which seek to simulate a Darwinian struggle towards the optimum solution of a mathematic problem have recently received attention and publicity. Here an initial population of solutions, with varying characteristics is pitted against one another in a computational survival of the fittest. Individual solutions can be combined to produce offspring and occasionally mutations are introduced. The environment is the problem that must be solved, and each possible solution is mathematically compared against this measure to determine its fitness. At the end of the calculation a single solution remains a mathematical entity best suited to the problem against which the computer was set. Modern computer scientists are through imitation, paying the sincerest form of compliment to the process that created living systems, and using its method to solve difficult problems.
In the nineteenth century scientists gradually became aware of the fact that life needs, in addition to basic nutrients which can be transformed into proteins and nucleotides, access to much smaller quantities of chemicals which organisms are unable to synthesize for themselves. The word 'vitamin' was coined to describe them, meaning vital amino or nitrogen containing compound; though it was apparent that their chemistry could be quite exotic. Soon it was discovered that there were in fact large numbers of trace compounds vital to life.
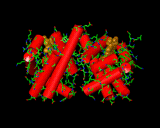
The intimate link between vitamins, trace elements and proteins was emphasized when crystallography came to be used to study the structure of these molecules of life. Often at the heart of the enzyme resides an active metal atom that is responsible for the activity of the protein. An excellent example is haemoglobin (left). This protein molecule contains iron atoms in a molecule that resembles a large disk. Iron held in this configuration is able to hold an oxygen molecule and transport it through the organism to centres which are able to exploit the oxygen molecule in the release of energy, enabling muscle movement or the synthesis of yet further molecules. The haemoglobin is beautifully adapted to its purpose. It exists in our blood stream in clusters of four molecules. When confronted with an oxygen rich environment this cluster is able to progressively adsorb large numbers of oxygen atoms. It achieves this by a process know as 'cooperativity'. The first oxygen atom absorbed distorts the protein slightly to make it easier for the next haemoglobin molecule of the group to adsorb the next oxygen molecule. Adding an item to the bundle opens up the carrier for subsequent sorbates until the package is full. This is how all packaging should be designed - but the opposite is often the case in the man-made world! This example emphatically illustrates how highly evolved the molecular mechanisms of life are. Although chemists can synthesize molecules based on iron able to transport oxygen, they are orders of magnitude less effective than the natural haemoglobin molecule, and we have the advantage of being able to steal ideas from the known protein structure. Nature has been able to set up an extremely efficient carrier system that is difficult to improve on. One might perceive this level of perfection to be discouraging, indicating - as often seems to be the case - that all the really good ideas have been taken. A slightly more optimistic viewpoint is that it illustrates the complexity of problems that can be overcome. If we can learn from the ways in which Nature handles perplexing problems then there is the prospect that our own most taxing problems, bacteria resistant to antibiotics or the elimination of environmental heavy metal contamination, for example, can be solved.
Many of man's most notable scientific advances have been made by a careful study, if not straightforward copy, of Nature. Pharmaceutically active molecules are very often taken directly from nature. After initial exploitation in their natural forms scientists may have learnt enough about their mode of action to make modifications to the molecule which may enhance its activity. Alkaloidal drugs provide an example of this exploitation followed by targeted modification. Morphine is a naturally derived product, extracted from poppy juice. Codeine and heroine are two well-known modifications of the morphine molecule, where small changes in chemical structure effect the way that the molecule is transported and treated within the body. The structures of these compounds are shown so that you can see the similarity between the molecules. Another well known example of the exploitation of a natural painkiller is salicylic acid, a compound extracted from willow bark. A slight modification of salicylic acid is acetyl-salicylic acid the drug that we know as aspirin. Many related compounds have now been developed and marketed, all related structurally to the salicylic acid molecule, and all binding to the same proteins within the body. We discuss more of the interaction of drug molecules with enzymes in Docking and Blocking.
Scientific knowledge of the molecules of life and the growth in preparative chemistry has exerted a strong influence on medical practice and pharmaceutical products during the twentieth century. At the turn of the century alkaloidal drugs were the most effective prescribed painkilling medicines. Effective antibiotic drugs included compounds of mercury, antimony, bismuth and arsenic, and often exhibited severely toxic side effects. As a result of the poisonous or addictive nature of many of the pharmaceuticals available in the nineteenth century, there was considerable empirical investigation of new classes of compounds for beneficial medical properties leading to the discovery of synthetic sulphur containing antibiotic molecules, improved sedatives and sleep aids, and superior anaesthetics which are in use to the present day. As advances in scientific knowledge have been obtained, pharmaceutical manufacturers have made corresponding adjustments to their research and development efforts. The determination of protein structure using crystallographic techniques has opened the way for the design of drugs targeted to specific receptors and active sites, a topic which is explored in Docking and Blocking, and an eventuality which has induced the majority of pharmaceutical companies to form macromolecular crystallography groups. In this world of the highly technical products the benefits of high quality research resulting in highly profitable products has been repeatedly demonstrated. In fact specializing in yesterday's pharmaceuticals rather than investing in developing technologies can be the riskiest option for today's drugs companies, as the demise of companies specializing in alkaloidal drugs at the turn of the century demonstrates.
In this section we have examined just a small number of the molecules of life, the ways in which we can understand their properties, and the ramifications of these properties. It has been necessary to select and highlight selected portions of the subject. The computer generated graphical representations used to highlight many of the structures, illustrate, in addition to the molecules depicted, the central role which the computer now plays in understanding experimental findings and allowing scientists to model the behaviour of these important molecules. When Dorothy Hodgkin and her coworkers set about solving the structure of vitamin B12 in 1960, the computer was a vital part of the process. The fact that the computer involved was in the United States of America whilst the crystallographic work was carried out in Oxford was dictated by the lack of any other suitable machine. In the 1960s computers were uncommon and required more coaxing than they do today to achieve a particular objective. They were programmed, in their own machine code, for specific tasks. Now a computer is part of the majority of chemical and biochemical experiments and their interpretation. The computers used by scientist worldwide are linked as part of a network that connects everyone. The resulting exchange of information and knowledge is propelling scientific discovery in the molecules of life.