Mon Oct 27 08:58:52 PDT 2008
The Material World and The Shapes of Crystals
As Madonna once said, 'We are living in a material world'.
And as Madonna indicates, occasionally it is interesting to reflect on the fact that the world around us is composed of molecules and atoms.
Indeed, the Greeks deduced the fact that substances must be composed of indivisible units called atoms in the sixth century BC.
However, it is sometimes hard to appreciate the importance and relevance of atoms and molecules in day to day life.
If you find yourself wondering about the immediate relevance of atoms in the world around you, take a look at a simple crystal. A diamond in a piece of jewelry, a grain of sugar, or salt has a very well defined shape. And that shape is determined by the structure of the atomic surfaces which make up that crystal.
Diamonds, sugar, and salt adopt their well defined shapes because of their regular atomic patterns. It doesn't matter how you make salt, it always makes crystals which are cubic. The well defined shapes of diamond, sugar, and all manner of crystals are a results of the well defined structures which their atoms adopt.
The image below is a view of the structure of a crystal of perovskite. Many compounds adopt the perovskite structure, including CaTiO3, BaTiO3 and MgSiO3. However, this is not an atomic level view, instead it is a view of the shape or morphology of a crystal of perovskite as you would see it in a sample of the real material.
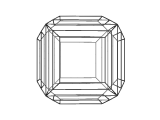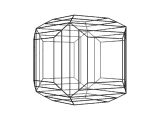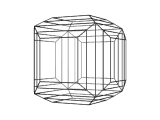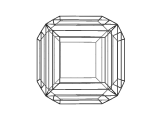
Using modeling techniques, and a knowledge of the crystal structure of perovskite, it is possible to calculate the morphology of the crystal.
Here is how atomic and molecular structure determines molecular shape. Atoms and molecules in crystals form regular packing arrangements based on inter-atomic forces. These forces cause the atoms of a given substance to always arrange themselves in a well defined structure.
However, crystals have a set of surfaces and these surfaces, for small crystals, are chosen such that the overall energy of the crystal is minimized. So the crystal favors surfaces with low energies. So the bulk packing of the atoms into the crystal and the surfaces which are seen in small crystals are those which minimize the energy of the system.
In our material world, diamonds, sugar, and salt, and all manner of materials, adopt their well defined crystal shapes as a consequence of their constituent atoms and the minimization of the energy of their systems.