Sun Dec 27 16:44:09 PST 2009
PETN (PentaErythritol TetraNitrate) A Molecule of Death
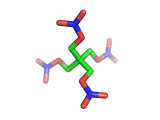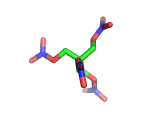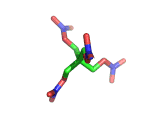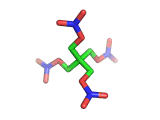
PETN, or PentaErythritol TetraNitrate, crops up in the news from time to time as a high explosive, often used by terrorists.
PETN's molecular structure is shown above. A central carbon atom supports four identical chains, each of which ends in a nitrate group. The resulting structure has the formula C(CH2NO3)4. This form of the formula emphasizes the four identical chains which the molecule contains. You can also write the formula at C(CO2)4(N2)24(H2O)4, which emphasizes the fact the PETN molecule contains just the right ratios of carbon, nitrogen, and oxygen to form many gas molecules from each molecule of explosive.
Not only do PETN's constuents contain a host of small stable combustion products ready to be released by the appropriate detonator, the molecule is also somewhat strained, with four identical large side chains terminating at the central carbon atom.
So when suitably triggered, PETN rearranges rapidly, releasing CO2, H2O, and N2, and plenty of heat, as these products are more stable than the PETN molecule itself. The result can be a devastating explosion.
Hence, PETN has been emplolyed by several terrorists, including Abdulfarouk Umar Muttalab.
Rather chillingly, when you look at the PETN molecule in three dimensions, as is shown above, at a certain orientation of the central tetrahedral carbon, the molecule takes on the shape of a swastika.