Atoms or What the World is Made of
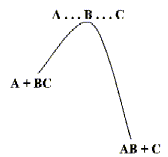 |
Atoms bond to form molecules because the energy of the resulting assembly is lower than the energy of the atoms in isolation. When two or more molecules meet, it is often the case that by changing their partners - by rearranging their bonding patterns - they can form more stable structures. The simplest example and one which is discussed in elementary chemistry classes is the reaction between H and H2. Such reactions rarely take place 'spontaneously'. There is usually some resistance to the rearrangement - an energy 'hill' that has to be climbed by the reacting molecules as shown on the left.
But once the molecules are 'over the barrier', the new chemical bonds form rapidly. Some of the most important types of chemical reactions are collected in Table below.
| Reaction Type | Example | Description |
| Electrophillic | H+ + C2H4 = CH2CH3+ | Protonation of an ethylene molecule |
| Nucleophillic | CH3Br + Cl- = CH3Cl + Br- | A substitution reaction: in this case a chloride ion (Cl-) substitutes for a bromide ion (Br-) |
| Free radical | O. + O2 = .O3 | An oxygen atom (O.) attacks and oxygen molecule (O2) to form an ozone radical (.O3) |
| Oxidation | 2Fe(s) + 3/2 O2 (g) = Fe2O3(s) | Iron (Fe) combines with Oxgygen (O2) to form iron oxide (Fe2O3). The (s) and (g) designations correspond to the solid and gaseous states of the species. |
| Reduction | 3C(s) + 2Fe2O3(s) = 3CO2(g) + 4Fe(s) | Carbon reacts with iron oxide to produce carbon dioxide and metallic iron. |
Reactions very often give out energy (although they may absorb it in some cases); the classical example is combustion where energy rich molecules react with oxygen to give low energy molecules - carbon dioxide and water, the basis of more modern fuels. There is a fascinating class of reactions that result in the emission of light - the field of photochemistry. And chemical reactions may also be used to create electric currents. indeed, batteries are nothing more than devices for changing chemical energy into electrical energy. And all these processes work in reverse. Heat, light and electricity may be used to stimulate and drive chemical reactivity. Indeed, the relationship between chemical reactivity and these physical phenomenon is one of the most fascinating ways in which the science of chemistry has developed in the last hundred years. We are constantly surrounded by chemical reactions. Our bodies rely on an enormous range of reactions to keep us alive, moving, breathing and thinking. Chemical reactions are developed and exploited by industry to make new products. At the heart of all these processes are molecules in which the atoms are rearranging and finding new partners. We have now established the ground rules for interactions between atoms.We have seen why they bind to each other and have learned something of the factors which limit and control the structures of the resulting atomic assemblies and of the way molecules interact.