Molecules and the Materials Below
 |
When molecules form chemical bonds with surface atoms, they modify not only the chemistry of the molecule (as we saw in the previous section) but also that of the surface and of the underlying crystal. Perhaps the most interesting effects relate to the electrical properties of materials. When molecules are adsorbed on surfaces, they may pull electrons out of the solid or put them in; in either case, they will alter the electrical conductivity of the solid. A good example is provided by the material tin dioxide (SnO2); when exposed to air, oxygen molecules are adsorbed on the surface of this solid. The oxygen molecules pull electrons out (making O2- ions) as illustrated on the left; the electrical conductivity of the material changes.
Suppose now we introduce an inflammable gas, like methane, into the atmosphere. The molecules of methane will also be adsorbed onto the surface where they will readily react with the oxygen (making carbon dioxide and water; they will 'burn' on the surface). The electrons which the oxygen molecules had pulled out of the solid will be returned and the conductivity will change back. We will see this immediately if we apply a voltage across the material, because the current will change. This rapid response of the current can therefore be used to detect the presence of the flammable gas; and tin dioxide is used as a sensor for flammable gases. Gas sensing of toxic and flammable gases is a growing field of applied science and one which is of obvious importance for environmental and safety concerns. Surface chemistry is clearly of central importance to sensing action.
Interfaces
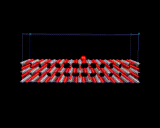
Surfaces of the type discussed in the previous section are interfaces between a solid and a gas (or possibly a vacuum). Other types of interface - between two solids and between a solid and a liquid and between two liquids or a liquid and a gas - are of course widespread in nature and have implications for many different scientific fields, including the study of corrosion, friction and capillary action; our theme here is the understanding of matter at the atomic level and the insights given by modern computational methods, we choose to highlight three examples of complex interfacial structures for which atomic scale models have been developed by computational methods. The first (shown beneath SnO2) is an example of an interface between a solid and a liquid, specifically between solid and liquid argon from a molecular dynamics simulation
 |
Our second illustration concerns a solid-solid interface, of a type that is being increasingly studied in several areas of current materials science. The image shows a model developed computationally of the interface between two oxide materials - aluminum oxide (Al2O3) and cerium dioxide (CeO2) - a system of importance because of the use of CeO2 on an Al2O3 support in automobile exhaust catalysts.
 |
 |
The model reveals the detailed way in which these two oxides cohere at the atomic level and allow us to understand the factors which control the stability of such interfaces.
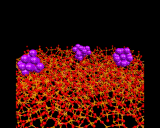
Our final example takes us back to the field of catalysis. It concerns the structures of metal particles on an oxide surface - a widely used class of catalyst. The image shows the calculated structure of a small particle of metallic nickel on the surface of silicon dioxide (SiO2). For the sake of comparison, we also show the structure of a 'free' particle of nickel of the same size. The difference is striking: when the Ni particle is placed on the surface, it spreads out - it 'wets' the surface. And this change in the surface structure of the particle will have profound consequences for the catalytic action of the metal.
 |
These illustrations show, once again, how with the aid of modern computational power, we are able to construct detailed models of highly complex atomistic structures. The science of surfaces and interfaces represents a major component of contemporary physical science. Next we consider the related but different structures occurring in the fascinating class of systems in which two surfaces are in close proximity.