Crystals
 |
Order, regularity and symmetry attract admiration in art, architecture and in the natural world. Of course, disorder and chaos characterise many types of physical entities; and the second law of thermodynamics formulates in precise and quantitative terms the evolution of all systems towards states of increasing disorder. But many natural objects are ordered and symmetric. Crystals have long been an object of fascination to the layman and to the scientist (especially so when rarity adds value, as in gemstones). Typical real crystals are characterised by regular shapes, smooth faces and sharp, well defined angles between the faces. We are also often interested and intrigued by the optical properties of crystals - their colour (or, as in diamond, their high refractive index which causes light to be reflected internally and results in the special glitter and brilliance of the stone); and mechanical behaviour, like the exceptional hardness of diamond, are another stimulant to our curiosity. The starting point of our enquiry in this section is, however, their special geometrical properties of regularity and symmetry. In particular, we will enquire whether the regularity of the external appearance of crystals is reflected in their atomic architectures.
The first clue to this puzzle comes from the experiment of Davidson. We recall how the wave-like properties of the electron were apparent from the results of shining a beam of electrons on a thin metal foil. The electron beam was 'diffracted' on passing through the foil, thereby showing both that the beam was behaving like a wave and that the arrangements of atoms in the foil was regular. Electrons interact strongly with matter; they can pass through, for example, very thin films, but cannot penetrate into the bulk of normal crystals. In contrast, another form of radiation, X-rays, (discovered , we will remember, by Röntgen in 1895 and which we now know is simply a form of electromagnetic radiation, like light but with a much shorter wavelength) will penetrate below the surfaces of solids. And the science of crystallography - the determination of the arrangements of atoms in crystals - was founded in 1912 by Lawrence Bragg, who discovered that crystals diffract X-rays. The only interpretation of this observation is that crystals consist of regular ordered arrangements of atoms which act as a diffraction grating; and by analysing the diffraction pattern, we can learn about the atomic arrangements in the crystal.
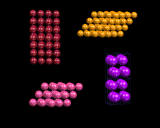 |
A crystal structure consists essentially of a 'pattern' of atoms (often referred to as the unit cell) which is repeated regularly and indefinitely in three dimensions, as shown in the images on the left. The pattern may be extremely simple, and in the limiting case may comprise just one atom, as in the crystal structures of many metals; structures in which the repeat pattern contains two atoms include sodium chloride (rocksalt) also shown on the left.
 |
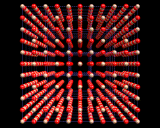 |
The other extremes are the crystals of viruses where the repeat unit is the whole virus containing hundreds of thousands of atoms. Yet, in one of the most remarkable achievements of recent crystallography, the crystal structures of viruses have been determined. The image on the left shows the structure of the 'foot and mouth disease' virus determined by David Stuart and coworkers.
So for these, the simplest of living things, we now know to a reasonable degree of accuracy the positions of all the atoms in their outer coats. This knowledge allows the design of anti-viral molecules which interact with the virus and interfere with the machinery of its protein armour.
 |