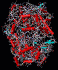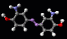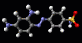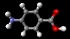Aspirin
 |
As we have seen the
previous sections, living entities are intricate
arrangements of macromolecular order and disorder. Each
protein is built from the blueprint of an organism's genetic
code. Proteins convert food molecules into energy,
assist in the transmission of information
through the organism, and defend against invading
parasitic cells. The molecular arrangements which
constitute life are highly complex and highly
interdependent. Governing these constituent processes is
a difficult task involving extensive chemical
communication. Electrical nerve impulses are triggered by
the molecular mediation of small molecules known as
neurotransmitters. Less pressing chemical messages are
carried by fluctuations in the concentrations of hormone
molecules in the blood stream controlling growth and the
sudden release of energy required in the flight from an
enemy, for example.
As humans have
evolved they have learned that, in addition to providing
food, some of the molecules made by plants and animals
have important effects on the body. Alcohol, for example,
known for thousands of years (an early product of the
biotechnology industry), variously prized and despised
for its effects on us, interacts with the normal passage
of nerve impulses in the brain. We call any chemical
which influences an organism a drug. Some drugs effect
the mood and behavior of their recipients and others
interact most strongly with invading organisms, killing
bacteria, for example, and leaving the
human healthy. Drug molecules interact with enzymes and
receptive molecular sites within an organism to
provoke or inhibit a particular response. The required
response might be the destruction of a bacterium, as in
the case of an antibiotic, or the inhibition of nerve
transmission in the case of a sedative. Understanding the
similarities between different molecules which achieve
similar effects can be a profitable way of exploring new
medicinal opportunities.
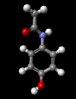
Paracetamol
 |
Ibuprofen
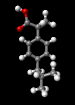 |
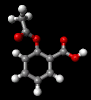
The structure of the
aspirin molecule is shown above with the
structures of ibuprofen and paracetamol. Aspirin is
derived from a compound found in the bark of the willow
tree and was discovered in 1899. The source of its pain
killing power is now known to be its interference with
the synthesis of a class of molecules, prostaglandins,
which cause inflammation in a variety of tissues, leading
to pain. The image above shows the three pain killing
molecules aspirin, ibuprofen and paracetamol, in a
spacefilling representation. We can see the structural
similarities of these molecules. All three have a six
membered ring structure, which tends to avoid the polar
environment of water and a comparatively small appended
group of atoms which are able to bond to part of their
receptor molecule. The similarity in the structure of
these molecules is clearly evident and from their similar
pain killing actions we can deduce that they can fit into
similar active sites even before the details of their
active sites have actually been determined. This
information can then be used to both understand the
activity of related compounds and also to help design new
compounds to analyze experimentally. In fact, the
structure of one of the receptor sites of these three
analgesic molecules has recently been determined using
the techniques of protein crystallography. Prostaglandin
H2 synthase-1 as this structure is known is a gigantic
molecule. It contains around five thousand atoms and
converts a long acid molecule, arachiodonic acid, into
inflammatory prostaglandin with its two distinct active
centers. The crystal structure of the receptor reveals
that the painkilling drugs need their common ring
structure to reach the active site of the molecule from
the non polar environment of the cell membrane. Once in
the active site the pain killers achieve their inhibition
of the formation of pain causing molecules through
differing interactions with the amino acid side chains of
the channel leading to the active site.
Arachiodonic acid.
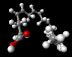 |
Prostaglandin H2 synthase 1
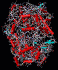 |
The early realization that synthetic chemicals could be
exploited in medical applications grew out of the use of
newly discovered molecular dyes in staining medical
specimens. These synthetic organic dyes, which had
revolutionized the textile industry, turned out to be
valuable in highlighting the differing regions of a cell
in microscope slide preparation. If dyes bound
differently to differing biological materials perhaps,
then, certain dyes and related compounds could target and
destroy bacterial cells. The impetus for a search for
compounds able to kill bacteria came, then, not from deep
conviction in Fischer's lock and key hypothesis, but from
pragmatic empirical observation. Paul Ehrlich was the
first chemist to pursue successfully this line of
investigation and through extensive preparative chemistry
and testing produced, in 1910, the arsenic based drug
Salvarsan, effective against diseases such as sleeping
sickness and syphilis. Although Salvarsan was used with
success in the treatment of a number of bacterial
illnesses, the majority of bacterial infections were
still without effective treatment. In the late 1920s the
German dye company I.G. Farbenidustrie (later to become
BASF, Bayer and Hoechst) employed Gerhard Domagk to
investigate the possible exploitation of its dyes in
medical applications. In 1932 Domagk discovered that one
particular dye, Prontosil, developed to stain leather,
was effective in curing mice infected with streptococcal
bacteria, and importantly, did not appear to harm the
animals, as many previously tested compounds had. Domagk
realized the importance of his discovery when his own
daughter contracted a streptococcal infection from
a simple puncture wound and was close to death.
Injections of Prontosil were effective in treating the
disease. Domagk reported his work in 1935 and sulphur
based drugs, as drugs of the Prontosil type became known,
were soon widely used. One early success was the treatment
of Franklin D. Roosevelt, Jr. the son of the President of
the United States.
Salvarsan
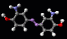 |
Prontosil
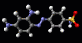 |
Like Ehrlich, Domagk decided to employ dye-like molecules
in his search for antibacterial drugs because
dyes exhibited differential staining
properties, leading to their routine use in microscopy,
implying that certain regions of an
organism, could be targeted at the molecular level.
Indeed, Domagk himself referred to the synthetic drugs
that he developed as 'magic bullets', able to target
selectively and kill a particular cell within a complex
living organism. How do such drugs achieve their
remarkable effects? The sulphonamide drugs inhibit
bacterial reproduction by interfering with the synthesis
of folic acid which then halts the production of the
bacteria's DNA, preventing the replication of the
bacteria. Bacteria unlike mammals must synthesize their
folic acid molecules in situ and this is achieved by
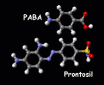
building up from para-aminobenzoic acid (PABA). Comparing
the structures of Prontosil and PABA shows that a part of
the drug molecule closely resembles the natural feedstock
chemical, and indeed the drug works by binding
permanently with the active site which might have
catalyzed the production of folic acid. Thus the
bacteria's synthesis of folic acid is blocked and
consequently bacterial reproduction grinds to a halt.
Folic acid is also vital for the human organism. However,
folic acid is not synthesized by humans, instead it is a
required part of our diets, it is a vitamin. Bacteria
cannot synthesize folic acid when Prontosil is present,
and they cannot make use of folic acid which might be
present in their host environment because bacteria have
not evolved a method which can transport folic acid
across their cell walls. Thus the sulphur containing drug
can kill the bacteria and leave the human unaffected - a
precise set of chemical interactions underlying the magic
of the medicinal bullet.
Folic acid
 |
PABA
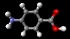 |
PABA and prontosil.
 |