Docking and Blocking
 |
The important ideas in science need time to gain acceptance. For example, since the late nineteen fifties that the precise images of crystallography have revealed details of the interactions between macromolecules and their molecular targets. It is easy, then, to conclude that the recognition of the importance of such interactions is a recent idea. Yet, in 1894, twenty years before the structure of sodium chloride was solved and more than half a century before any protein structures were known, Emil Fischer, a Dutch organic chemist, was able to propose that enzyme and substrate fit together 'like a lock and key'. Fischer's bold proposal was made after careful study of the effects of enzymes on sugars and explained the precise specificity of enzymes able to distinguish between even the mirror images of the same molecular structure.
Fischer's hypothesis withstood scientific scrutiny, inspired Linus Pauling in his pioneering model building as molecular biology began, and is now a central theme in the rational design of drugs. As we examine the precise registry between molecular entities through computer graphics images in this section we should not lose sight of the fact that the closeness of the interactions involved was first understood more than one century ago. We will see the ways in which molecular recognition, as the specific intimate interaction of molecules with one another is known, influences all forms of microscopic chemical communication.
Molecules have distinctive shapes and correspondingly distinct properties. The realization that chemicals were three dimensional entities came about in the nineteenth century through the clear reasoning of physical chemists like Le Bel and van't Hoff. Indeed, van t'Hoff's successful models, proved to be the inspiration for Fischer's explanation of an enzyme's ability to differentiate, at the most subtle level, between molecular shapes, yielding the original lock and key hypothesis. Although this framework for understanding enzyme activity has now been in place for one hundred years it has taken time, many experiments and analysis to add detail to the intuitive understanding of the pioneering biochemists.
Naturally, there are good reasons for the gradual adoption of three dimensional chemical reasoning. The most important is that although special properties could be explained on the basis of unique shape - direct evidence of the shapes involved was difficult to obtain. The small size of molecules renders the determination of structures difficult. Indeed, molecular structure is still often difficult to determine in many circumstances and in the past this limitation has been even more pervasive than it is today. The advantages that three dimensional structural information bestow have become progressively more apparent as molecular biology and structural chemistry have advanced adding impetus to the drive to determine yet more stuctures. An interesting example of the delightful insights of stuctural information is provided by the history of the study of the enzyme lysozyme. This example again highlights the opportunities afforded by the interaction between differing fields of scientific research. Medicine, chemistry and crystallography collided to provide the first glimpse of Fischer's molecular 'lock'.
 |
The medical need for improved treatments for bacterial infection began the chain of events. Throughout the majority of our history bacterial diseases have been lethal to humans. Today, however, such infections can be routinely treated with antibiotics. Our modern exploitation of antibiotics began in 1929 when Dr. Alexander Fleming, working at St. Mary's Hospital Paddington, discovered penicillin, a compound excreted by the penicillium strain of mould accidently growing on a bacterial culture.The search for antibiotic compounds was, however, a long one, and its discoveries were to influence enzyme science in addition to therapeutic medicine. In 1922 Dr. Fleming discovered that lysozyme, an enzyme constituent of nasal mucus, was active against bacterial cultures. Although Lysozyme, named because of its ability to dissolve or lyse bacteria, had pronounced anti-bacterial qualities, it was not effective against the most lethal forms of bacteria. Lysozyme, however, was found in, and excreted by, many living tissues, including the tears of volunteer donors. Eventually a particulary convenient source of the enzyme was found in the white of hens eggs. Unlike many protein molecules, Lysozyme also proved to be comparatively straightforward to extract, purify and crystallize and for this reason proved to be an excellent candidate for the emerging science of macromolecular crystallography. Importantly, too, it was possible to prepare crystals with differing heavy atoms incorporated into the structure in the same position, making it possible to reconstitute from diffracted X-ray reflections accurate maps of the electron density of the atoms of the lysozyme molecule.
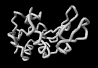 |
In 1962 Lysozyme, then, became the first enzyme structure to be determined. The lysozyme molecule is large in comparison with many of the molecules you will see on this site, containing around 1950 atoms. The determination of their electron density map from hundreds of diffracted X-ray intensities was an astonishing feat. The work was carried out at the Royal Institution of Great Britain by David Phillips and coworkers. The Royal Institution has seen many scientific break throughs. It was at the Royal Institution that Davy and Faraday pioneered modern chemical and physical research and both William and Lawrence Bragg, the discoverers and pioneers of X-ray structural anlysis, were Laboratory Directors.
 |
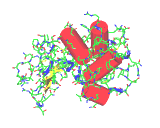
The images on the left are of a single molecule of the enzyme lysozyme. The molecules contains 1950 atoms. The upper image shows all the non-hydrogen atoms of lysozyme - this image shows the molecular shape - but it is hard to see the overall architecture of the molecule.The image to the left conveys the shape of the lysozyme molecule with a solid ribbon following the polypeptide backbone. Below we show an image where the alpha helical portions of lysozymes are highlighted with red cylinders.
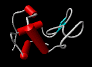 |
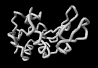 |
This initial structural discovery was followed by the structural complexes between the enzyme and bound saccharide substrate. Lysozyme catalyzes the destruction of bacterial cell walls through cleaving a particular link between two sugar molecules. Understanding this reaction illustrates the accuracy of Fischer's lock and key hypothesis and also shows how the enzyme is able to bias the natural reactive tendancy of a given molecule or set of molecules to achieve a particular effect. The enzyme is a natural catalyst. Just as the catalytic converter of a modern car increases the rate at which the undesirable products of internal cumbustion are combined with oxygen and hydrogen to yield less harmful chemicals, so the enzyme increases the rate at which a given chemical transformation is achieved within a living organism. In the case of lysozyme the reaction catalyzed is the hydrolysis, or break down by adding water, of the linkage between two sugar in a chain of sugar molecules. This reaction might also be catalyzed using a strong acid. Indeed, this is precisely the way in which starch may be converted into useful sugar molecules industrially. But concentrated acids are corrosive and difficult to handle even in the laboratory and within a living organism will indiscriminately attack the vital structures of the host organism, such as nucleic acids and protein molecules. What a living organism needs is a catalyst specifically tailored to fit the desired target or 'substrate'. Molecules which precisely fit the active site of the enzyme lysozyme find themselves acted upon by a powerful acid catalyst. Molecules which do not fit the lock are safe from destruction. The enzyme may even 'squeeze' the saccharide molecule, in a molecular sense, to promote the formation of the desired breakdown products. Other molecules, DNA or hormones for example, do not fit the lysozyme active site and thereby avoid the acid mediated destruction which befalls the bacterial cell wall.
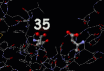
The registry of the lysozyme molecule and the bacterial saccharide molecule is highlighted in the image on the left. This image also reveals the residues, the specific amino acids, responsible for lysozyme's activity. The first active amino acid is an acidic group, aspartame the thirty fifth amino acid of the lysozyme chain, coloured yellow in the diagram. This amino acid is placed on one side of the binding groove occupied by the saccharide chain in the image on the left. Residue thirty five is surrounded by unreactive amino acids leading researchers to believe that at the normal operating conditions for the enzyme it is this amino acid which supplies the acidity necessary to break the linkage between two sugars molecules. On the opposite side of the enzyme's cleft is a negatively amino acid side chain (which is also highlighted in the diagram). When the sugar is attacked by the acid it becomes positively charged and this second side chain stabilizes the resulting molecular entity until it can rearrange to the breakdown products of the catalytic reaction. It is the cunning molecular architecture of the enzyme which stabilizes the transition from intact to cleaved bacterial cell wall and promotes the destruction of a foreign cell.
The careful experimental investigation of the catalytic properties of the lysozyme molecule heralded unparalleled rationalization of a range of catalyic mechanisms. For the first time it was possible to understand the molecular constraints and appropriately poised functional groups used by nature to build and destroy molecules, cells and organisms. Many other catalytic mechanisms have now been exposed by careful experimental investigation and crystallographic work at a similar level of atomic detail. Fischer's lock and key hypothesis has been shown to be extremely accurate. As we shall see, the key-like interaction of a molecule with a receptor site on a macromolecule has also turned out to be an extremely valuable way to understand the properties of many molecules even when no direct information exists about the actual structure of that receptor.
The structures revealed by today's experimental techniques are averages representing many molecules and their positions over long periods of time. However, a static view of the entities is an over simplification. Molecules must diffuse to and from the active site, often carried by a dynamic sea of water molecules. Careful measurement of the rates of enzymatic reactions and protein folding have long indicated the existence of considerable flexibility in even fully formed enzymes structures. Indeed, computer simulations also highlight the flexibility of Fischer's molecular locks as they envelope and appropriately embrace their molecular keys. The image on the left shows superimposed frames from a simulation of a substrate in the active site of lysozyme over a period of just 10-12 seconds.