Tue Nov 25 21:04:48 PST 2008
Recycled Designer Water
Water, water, every where,
Nor any drop to drink.
Or perhaps that should be, all of it fit to drink. The space station astronauts will soon be enjoying refreshing, recycled water. This will enable the space station to support a larger number of occupants with the same amount of water. Additionally, there will be a reduction in the amount of effluent which needs to be handled to keep the space station operational.
While the recycling of water might seem a desperate measure, it is worth remembering that virtually all the water on earth has been 'used' several times. Those few water molecules that haven't been 'used', have probably just been created by the combination of hydrogen and oxygen atoms, and even those atoms have probably been through many cycles themselves.
So water re-cycling is nothing new.
Here are some of the things that may have happened to the last water molecules that you consumed:
- They may been consumed and excreted by Caesar
- They may have arrived on earth as an important constituent of a huge comet which collided with the earth billions of years ago
- They may have arrived on earth as a major constituent of a 'main-belt comet'. Such comets continually collide with the earth's atmosphere depositing water molecules on the planet
- They may have formed part of a mineral deep within the surface crust of the earth only to be 'out gassed' by volcanic activity
- They have been formed by the combustion of hydrocarbons, even by environmentally unfriendly cars, in reactions such as this CH4 + 2O2 -> CO2 + 2H2O
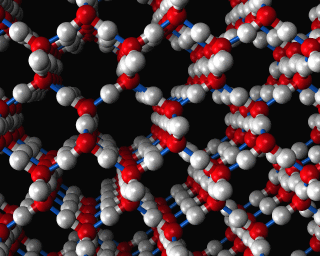
A water molecule has two hydrogen atoms and one oxygen atom. Each water molecule can participate in 4 hydrogen bonds, two involving the oxygen atom and one for each of the two hydrogen atoms. (The image above shows hydrogen bonds between water molecules in ice, the hydrogen bonds are shown as light blue cylinders.) This network of hydrogen bonds for each water molecule gives rise to distinct three dimensional structures, such as the structure of ice shown above. The hydrogen bonds make the forces between water molecules strong. Consequently, water is a liquid at room temperature, while similarly sized molecules (like methane for example) are gases.
On the surface of water, the strong forces hold molecules rigidly in place, giving rise to surface tension. In turn, the surface tension of water gives rise to the liquid properties that we rely on, from the size and shape of water droplets, to our ability to improve water's wetting ability with detergents.
Occasionally, the marvels of simple water molecules and the structure of liquid water inspire people to become carried away with molecular flights of fancy. So, for example, enterprising individuals sell bottles of 'special' water, in which, it is claimed, unique clusters of water molecules are present. However, although you would not know it by the sales of such products, it is impossible to have water molecules do your bidding in a bottle. Water is chaotic water. Even the expensive bottle of designer water, with the 'specially produced water clusters for extra hydration' is just a bottle of recycled molecules that has been filtered through several organisms and possibly your neighbors on its way to its fancy bottle!