| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| 6 | 7 | 8 | 9 | 10 | 11 | 12 |
| 13 | 14 | 15 | 16 | 17 | 18 | 19 |
| 20 | 21 | 22 | 23 | 24 | 25 | 26 |
| 27 | 28 | 29 | 30 |
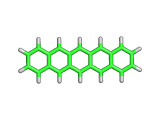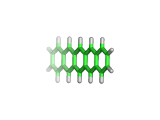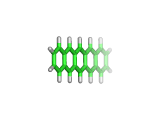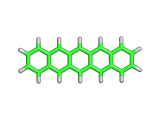
As if there were every any doubt, researchers at IBM have recently created beautiful direct images of the pentacene molecule using an Atomic Force Microscope (AFM).
Pentacene, as you can see above, contains five six-membered carbon rings in a linear chain together with their associated complement of hydrogen atoms. Pentacene is an aromatic molecule with interesting properties. Somewhat like graphite, and yet truly molecular, pentacene has electrical conductivity like graphite, and the controllability of a molecular organics. Hence there is great interest in putting pentacene to work in molecular electronic devices.
The atomic resolution of the atom positions in pentacene will come as no surprise to crystallographers. Crystallographers have been able to 'see' atoms for close to 100 years. However, crystallographers need crystals. Without a regular array of diffracting atoms, conveniently provided by nature in the form of crystals, crystallographers cannot carry out the their trade. So the atomic resolution of the atoms in a single molecule is, strangely, big news. The AFM, one day, may allow chemists to characterize individual molecules simply by tracing their connectivity. Perhaps one day it will be possible to map the binding site of a protein, or the key interaction regions of an antibody, on a molecule by molecule basis, using an AFM.
The IBM image, which you can see here, is brighter at the ends of the molecule. It is tempting to speculate that this is a result of the AFM directly sampling the molecular orbitals of the pentacene, rather than simply its electron density. Perhaps experiments are finally catching up with quantum mechanics, which have been able to see molecular orbitals for many decades!
For a while we will probably see AFM images confirming what has long been established through crystallography and quantum mechanics. However, as experience with the technique increases, and interest in vanishingly small states of matter accelerates, we will undoubtedly see surprises as scientists get up close and personal with individual molecules.