Sat Sep 13 11:57:23 PDT 2008
Triacetone Triperoxide (TATP)
Welcome to the The Molecular Universe site! (For information on ecstasy and heroin follow the appropriate links).
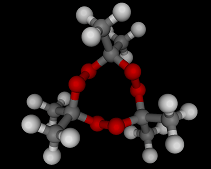
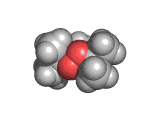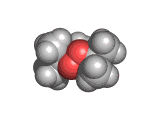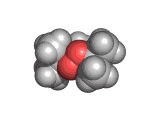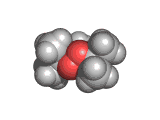
The molecules above and below are both triacetone triperoxide (TATP). The rotating image above shows the ring structure of the TATP molecule and the static image below shows the symmetrical way in which TATP packs in three dimensions.
Triacetone triperoxide, which is also known as TATP, is a ring of three acetone molecules, joined together by -O-O- linkages. These linkages are peroxy linkages. Molecules which contain peroxy linkages are generally keen to release oxygen, because the oxygen-oxygen bond is not strong and the released oxygen atoms are reactive and attack nearby molecules. Hence, the bleaching and oxidizing effects of hydrogen peroxide, which attacks the color giving molecules in hair or furniture making them lighter in color.
Not only are peroxides like TATP effective oxidizing agents, they are chemically unstable and when suitably energized, rapidly return to their more stable chemical constituents. Hence, hydrogen peroxide, for example, gradually decomposes to water and oxygen.
Left to its own devices, TATP can decompose to oxygen or ozone and acetone. However, TATP is so unstable that it can spontaneously explode in an unpredictable and catastrophic manner.
TATP is especially dangerous because it is constrained in a ring like structure, the carbon and methane groups which are shown in gray and white in the images below do not allow for the elimination of the unstable extra oxygen atoms and the retention of the ring like structure at the same time. When the rings of TATP fall apart the rearrangement is necessarily wholesale and dramatic.
The mixing solvents like acetone and peroxides like hydrogen peroxide happens mistakenly on occasion in laboratories. On this occasional basis the inherent and dangerous instability of TATP is rediscovered inadvertently and explosively in a refluxing vessel or a when a precipitate is being dried in an oven. If you work in a laboratory, be very careful when working with peroxides.
The Molecular Universe provides interesting and accessible information on molecules and molecular systems. Take a moment to investigate the material and molecular world that surrounds us on The Molecular Universe site!