| Su | Mo | Tu | We | Th | Fr | Sa |
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| 7 | 8 | 9 | 10 | 11 | 12 | 13 |
| 14 | 15 | 16 | 17 | 18 | 19 | 20 |
| 21 | 22 | 23 | 24 | 25 | 26 | 27 |
| 28 | 29 | 30 |
It is hard to beat the beauty of C60, also known as buckminsterfullerene. This is the molecule you see below these words.

The C60 molecule was discovered by chemists and physicists examining the bonding and spectroscopy of carbon in its various forms. The fact that their experiments showed anomalously stable groups of carbon atoms around certain 'magic' numbers of atoms came as a surprise. Soon the investigators began to suspect the existence of a new form of carbon, not based on continuously bonded arrays of carbon atoms, as in graphite and diamond, but based on groups of carbon atoms clustered to form molecules.
Once enough C60 had been collected and purified its structure could be unambiguously determined.
And the C60 structure that you see above did not disappoint. It is highly symmetrical and pleasing to behold - and somehow this is what one expects of important molecules.
Does the molecule below look like anything else in The Molecular Universe? Probably not, it is pretty unique. But does it look like anything at all? Well, it is quite clearly a human figure. It is a real molecule, synthesized and characterized by Stephanie Chanteau and James Tour from Rice University in Texas, and called 'NanoKid' by its creators.
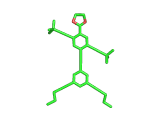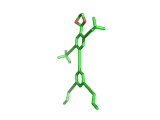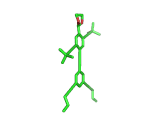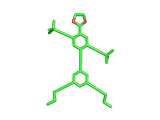
Actually Stephanie and James synthesized a whole range of molecules, all resembling people, demonstrating that chemists can create virtually any molecule that they set their minds to. You can read about their work here.
If you have ever connected a molecular modeling kit together to create animals or arbitrary shapes, you will know that molecules make for potentially wonderful 'tinker' toys. Organic chemists get to play with real molecule sized molecules and make their special creations for real on the molecular scale.
The paper describing this work is titled 'Synthesis of Anthropomorphic Molecules: The NanoPutians' which shows imagination. It is the control over molecular architecture that Stephanie and James demonstrated in their work impresses you most, though. Undoubtedly, this level of molecular control will one day contribute to the creation of nanoscale electronic devices. And it is not surprising to learn that Stephanie and James are based at a center for Nanoscale Science.
It may take years, but one day there may be molecule sized switching elements at the heart of electronic devices. When and if that day comes, the computers that make use of such devices will have extraordinarily high numbers of computing (and memory) elements per unit volume and have reasonable power requirements, simply because molecules are so small.
And it is possible that some of the techniques used to create NanoKid will be used to make those electronic devices of the future.
(By the way, hydrogen atoms in the image above have been suppressed. This keeps the image in line with the original publication and this is the way the organic chemists normally look at their molecules. In the case of the NanoKid molecule it also keeps the pelvis region unadorned...).
More information on topical molecules in The Molecular Universe.
Oxycodone (shown below) is a drug which acts on the brain in much the same way as heroin or morphine. Oxycondone is marketed as OxyContin (the 'Contin' part of the name indicating continuous release) and when mixed with an analgesic the resulting combination is marketed as Percocet, Endocet, Tylox, Roxicet, Percodan, Endodan or Roxiprin.
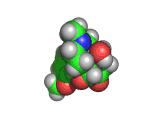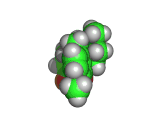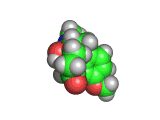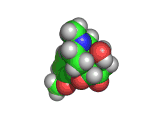
Comparing the structures of oxycodone and heroin you will see many similarities. The receptors in your brain are similarly hard pressed to distinguish between the two molecules and the effects of oxycodone are very similar to the effects of heroin.
Medically oxycodone is a powerful pain killer and this is what the drug OxyContin is prescribed for. However, like heroin, which is also an effective pain killer, oxycodone is highly addictive.
This has led to many people originally prescribed oxycodone for their pain becoming addicted to the drug. Additionally, like heroin, oxycodone suppresses respiration making it quite possible for casual users to overdose and to be asphyxiated by the drug.
Naphthalene is a simple molecule. It contains just ten carbon atoms and eight hydrogen atoms. You can see its structure, both in two dimensional chemist's sketch form and in three dimensional space filling form below. (The hydrogen atoms are not shown in the two dimensional sketch - their positions are infered on the basis of the number of bonds that the carbon atoms possess).
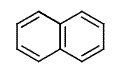
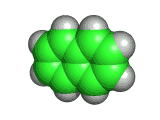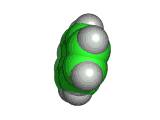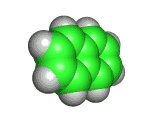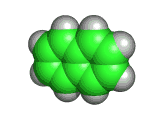
Although naphthalene is simple it is useful. It has long been used to repel moths and other insects, and sold for this purpose in the form of mothballs. And, surprisingly, despite naphthalene's long use, there have been few sensational scares concerning its toxicity to humans. However, naphthalene is not something any human should consume - and in fact eating mothballs or inhaling large amounts of naphthalene will do you no good at all.
Naphthalene was one of the first organic compounds to be examined using x-ray crystallography. William Bragg published the structure of naphthalene in 1921, nine years after his son, Lawrence Bragg, had shown how diffracted x-ray intensities could reveal the atomic structure of crystals. Now x-ray crystallography is used to determine the structure of molecules with thousands of atoms. However, in 1921 the detailed structure of a molecule such as naphthalene was extraordinary. For the first time, chemists could see in great detail the structures of molecules that they were working with in the laboratory. The 1920s also saw developments in quantum mechanics and soon both these branches of science were able to interact. Quantum mechanics provides predictions and explanations for the observations of crystallography.
Naphthalene, despite its simplicity, continues to be of interest. It is useful as an intermediate for the construction of more complex molecules. Naphthalene is found in meteorites and coal. Recently it has been found by astronomers in the interstellar medium, the very diffuse clouds of material and molecules between stars.
So when you smell naphthalene, which is quite recognizable given its use as an insect repellant, remember that it was one of the first organic molecules to have its structure determined by crystallography and that there are vast numbers of naphthalene molecules between the stars!
Melamine, the molecule shown below, has the formula C3H6N6, and the chemical name 1,3,5-triazine-2,4,6-triamine. It is an aromatic molecule; it possesses a six atom ring of carbon and nitrogen atoms with delocalized bonding increasing the stability of the ring.
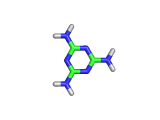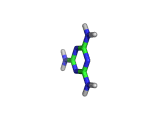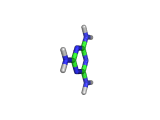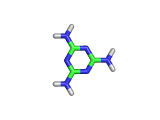
Melamine and formaldehyde are the main components of the plastic known as melamine resin. The polymerization occurs when melamine and formaldehyde are baked together. Melamine resin is the plastic coating of particle board based furniture.
As you can see from the formula, this molecule contains many nitrogen atoms. This gives rise to two additional applications of melamine, the first of which is generally beneficial, and the second potentially lethal.
The first application is as a flame retardant. Melamine is added to many plastics and other materials. When the melamine molecule decomposes, it releases nitrogen. As nitrogen is a relatively inert molecule, the nitrogen that is released tends to smother flames caused by the oxidation of the plastic. Hence melamine serves the purpose of an emergency supply of an inert gas to be called upon as the polymer is beginning to thermally decompose.
The second application is as a food adulterant added to increase the apparent nitrogen content of various food sources. This illegal application makes use of the fact that melamine is a low cost source of nitrogen, and that many tests for protein content actually simply measure the nitrogen content of the food. The resulting food adulteration has had terrible consequences as melamine is a toxic molecule. Many humans and animals have suffered and even died as a result of consuming melamine tainted foods.
There are a variety of solutions to the melamine in food scandals. More sophisticated tests for protein content could be used; specific adulterants like melamine could be tested for, penalties for selling adulterated foods increased, or chief executive officers given a pivotal role in proving the quality of their products by using those products themselves!
Melamine itself is just a collection of nitrogen, carbon, and hydrogen atoms. Its application in plastics stem from its ability to make three bonds to other monomers and its ability to quench fires with its nitrogen atoms. Its deleterious applications owe their origin to the willingness of criminals to exploit a molecule's properties in order to trick their customers and the public.
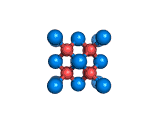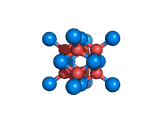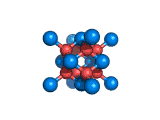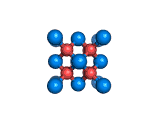
The structure shown above is a small fragment of the fluorite crystal structure. This is a crystal of calcium and fluorine that repeats through space based on the building block which is shown above. The formula of fluorite is CaF2; there are twice as many fluorine ions as calcium ions in the structure. The fluorine ions occupy tetrahedral holes in the lattice of calcium ions. In the image above, the fluorine anions are shown in red. (There seems to be an ancient tradition of displaying negatively charged portions of materials and molecules in red) and the calcium ions are shown in blue (again there is a tradition of coloring positively charged portions of chemical structures in blue).
Fluorite, which is also known as fluorspar, is found in crystalline form in nature and is the feedstock for the product of hydrogen fluoride which is an important industrial chemical. Fluorite crystals are often octahedral in shape.
Two other materials which can occupy the same structure as fluorite are uranium dioxide, UO2, and zirconium dioxide, ZrO2. Uranium dioxide is used in the nuclear industry and zirconium dioxide is used in oxygen sensors. In both materials, vacancies on the anion lattice (the red spheres in the image) allow a certain degree of mobility for ions within their crystal structures. This anion mobility gives rise to some of the interesting properties of fluorite structured materials.
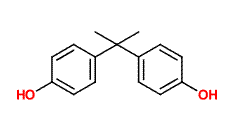
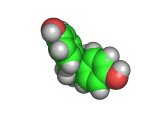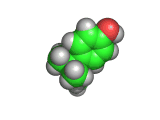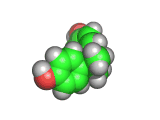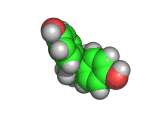
The molecule shown above is bisphenol-a, also known as BPA. Like TATP (triacetone triperoxide) the bisphenol-a molecule is synthesized by allowing acetone to react with other molecules. In the case of bisphenol-a the other molecules are phenol. When catalyzed by an acid (as is also the case in the TATP reaction), two phenol molecules react with one acetone molecule, eliminating water and creating bisphenol-a. The 'a' in this molecule's name is simply taken from 'acetone' showing just how unsystematic common chemical names can be.
Bisphenol-a is used as an antioxidant for the plasticizers in many plastic and polymer products and also inhibits additional polymerization, which leads to brittleness in some plastics. This compound is made in large quantities by the chemical industry and is used in many of the products in your home as a polymer additive.
With its two aromatic rings with hydroxyl groups, bisphenol-a is able to act as an antioxidant by combining with oxygen containing radicals which would otherwise attack the carbon backbone of the plastic. Once the bisphenol-a reacts with the radical, the radical electron is delocalized and stabilized by the aromatic rings of the bisphenol-a molecule and this stops what might otherwise be a chain reaction. The ability of bisphenol-a to react with radicals in this way is a similar to that of vitamin E, which is occasionally added to foods to prevent free radical induced spoiling.
Bisphenol-a has been used in plastics which come into contact with food for many years and while the small quantities of bisphenol-a that reach humans as a result are tiny there is some doubt as to whether bisphenol-a is an entirely safe additive. It has to be said that many molecules reach us from many sources, and even vitamin E should not be taken in substantial doses. So while the concerns about bisphenol-a should not be dismissed, and should be carefully researched, they are also not something to worry about unnecessarily.
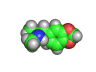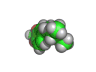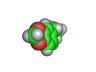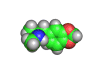
In the 1960s and 1970s interest in psychedelic drugs increased and this led to the realization in some circles that there was money to be made in selling molecules capable of producing altered states of consciousness in the people who consumed them.
At the same time, a variety of relatively simple chemicals from natural sources, like mescaline from cacti, were known to cause hallucinations.
So the combination of large sums of money and increasing chemical knowledge created a unique set of conditions in the 1970s and 1980s.
Back street chemists soon discovered that relatively easily created molecules were also psychoactive compounds. Hence 'designer drugs' were born. These molecules were and still are dangerous. Ecstasy, which is shown above, has been linked with nerve damage in animal studies. MPTP (1-methyl-4-phenyl-4-(1,2,3,6-tetrahydrpyridine)), caused terrible brain damage in people who took the compound intravenously. The victims of MPTP have been left unable to talk and paralyzed. This is a terrible price to pay for cheap chemical euphoria.
Anyone taking any form of illicit drug needs to recognize that they are ingesting materials which have a very uncertain provenance. The people who synthesize such compounds are clearly not overly fussy about purity or preparatory niceties. A typical street drug will be contaminated with solvents and by products and will have been diluted with almost any material in the 'cutting' process.
Ecstasy takers risk permanent neurological damage. The long term effects of ecstasy are not yet fully characterized, though animal studies have indicated that nerve damage is a real possibility. The action of the drug is not understood either. There is currently a theory that ecstasy produces a release of oxytocin; and it is this natural hormone which produces the increased feelings of bonding and relatedness for which ecstasy is famed.
So, those interested in the effects of ecstasy, and also interested in being green and not encouraging criminal activity, might try finding someone to hug. (Apparently hugging produces an outflow of oxytocin into the blood stream).
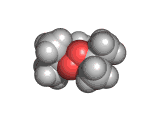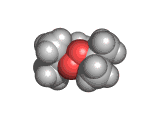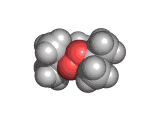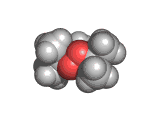
Welcome to the The Molecular Universe site! (For information on ecstasy and heroin follow the appropriate links).
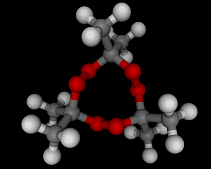
The molecules above and below are both triacetone triperoxide (TATP). The rotating image above shows the ring structure of the TATP molecule and the static image below shows the symmetrical way in which TATP packs in three dimensions.
Triacetone triperoxide, which is also known as TATP, is a ring of three acetone molecules, joined together by -O-O- linkages. These linkages are peroxy linkages. Molecules which contain peroxy linkages are generally keen to release oxygen, because the oxygen-oxygen bond is not strong and the released oxygen atoms are reactive and attack nearby molecules. Hence, the bleaching and oxidizing effects of hydrogen peroxide, which attacks the color giving molecules in hair or furniture making them lighter in color.
Not only are peroxides like TATP effective oxidizing agents, they are chemically unstable and when suitably energized, rapidly return to their more stable chemical constituents. Hence, hydrogen peroxide, for example, gradually decomposes to water and oxygen.
Left to its own devices, TATP can decompose to oxygen or ozone and acetone. However, TATP is so unstable that it can spontaneously explode in an unpredictable and catastrophic manner.
TATP is especially dangerous because it is constrained in a ring like structure, the carbon and methane groups which are shown in gray and white in the images below do not allow for the elimination of the unstable extra oxygen atoms and the retention of the ring like structure at the same time. When the rings of TATP fall apart the rearrangement is necessarily wholesale and dramatic.
The mixing solvents like acetone and peroxides like hydrogen peroxide happens mistakenly on occasion in laboratories. On this occasional basis the inherent and dangerous instability of TATP is rediscovered inadvertently and explosively in a refluxing vessel or a when a precipitate is being dried in an oven. If you work in a laboratory, be very careful when working with peroxides.
The Molecular Universe provides interesting and accessible information on molecules and molecular systems. Take a moment to investigate the material and molecular world that surrounds us on The Molecular Universe site!
The molecule below is heroin. Although it looks innocuous, the addiction that heroin produces causes a great deal of suffering. Heroin is derived from the natural opiate morphine. The chemical change that creates a heroin molecule from a morphine molecule is the transformation of two -OH groups into two -CO-CH3 groups. This change leaves the heroin molecule better able to penetrate the blood brain barrier and therefore to get to the receptor sites which allow it to exert its influence.
Interestingly, by the time they interact with the receptor site, both morphine and heroin are identical. The -CO-CH3 groups are stripped off in the body and the heroin molecule reverts to being a morphine molecule.
The morphine molecule binds strongly to a receptor which would normally interact with peptide hormones. These are the molecules which your body might produce during a briefly euphoric event, such as surviving a foot race with a fearsome predator. However, this receptor was not designed to deal with a foreign molecule such as the morphine molecule and it is swamped by the presence of this molecule. Although morphine is a natural product (it is, of course, produced by poppies among other plants) it is by no means good for humans and when made even more able to cross the blood brain barrier than it natural ancestor by the addition of those -CO-CH3 groups, its affects are even more pernicious.
You can read more about drug molecules and their receptors in Docking and Blocking in The Molecular Universe site.
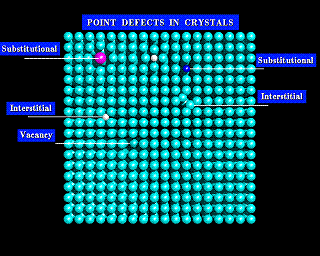
The images below show two models of a simple solid. The first model is not labeled, and at a first glance looks regular. However, if you look closely you will see a number of imperfections in its structure. These defects are labeled in the second image.
Why the interest in imperfections? (You might well ask!). Just as for people, imperfections make solids interesting. Defects give solids many of their most important properties. If you are reading these words on a computer, you are reaping the benefits of defects in silicon. Defects make silicon a controllable semiconductor that can be used to make transistors and integrated circuits.
The simplest types of defects are vacancies; atoms that are simply missing from their normal sites. There are also substitutional defects, where a foreign atom occupies the site of a given atom within the lattice. Finally, there are interstitial defects. Interstitial defects are atoms between the normal lattice sites of the crystal. You will see each of these defects in the diagrams below.
Defects control many of the properties of alloys, semiconductors, and all manner of materials. Imperfections are also implicated in the corrosion of metals, catalysis on surfaces, and the growth of crystals. So, defects are important!
You can find more information on defects in The Molecular Universe site.
I started this blog to document developments to The Molecular Universe site. I thought that it might be useful to provide background to the main site: overviews of some of the articles on the main site, topical information on Molecules and Materials, and also information on the way that the whole site is constructed, that sort of thing.
The reason I was interested in documenting the construction of the site is that when you set out to create a web site there is not all that much information around on how to go about the task. Or perhaps it would be more accurate to say that there is relatively little focused information. There is plenty of information but it scattered in many places and it is generally not collected near the site that it represents. There are many software products, environments, productivity tools, and so on. While these are, no doubt, wonderful, they are also typically proprietary or constraining. They make everything straightforward as long as you can stick to a single user interface and way of doing things. If there are bugs or issues to be fixed, you may be left waiting for the next release. If you want to experiment with PHP, AJAX, or a similar technology, and your chosen environment does not support that capability, then you will need to upgrade or migrate to a new technology.
Instead, I wanted an ultra simple site. I wanted to be able to focus on the wording and images and incrementally improve the quality of text with time. As the pages and images are interlinked, I wanted the busy work overhead to be as low as possible. I wanted PDF files to be generated for any page on the site. The reason for the PDF files was to make life simple (and predictable) for anyone that wanted to read a section of the site offline.
So, I decided to make use of the following basic technologies, and nothing more, in the construction of The Molecular Universe site.
HTML is used to author the articles. This presents a substantial danger in that content may be inextricably mixed with 'style' information throughout the text. In order to minimize this risk, I ensure that the HTML is well formed using 'tidy'. Hence, if I want to drop a subset of HTML tags, I can easily do this. If necessary, I can slim the pages down to raw text and minimal formatting information covering subscripts and superscripts, for example.
PDF The PDF files themselves are generated from the HTML. There is no need to use a manual process to create PDF files, going through a word processer, for example.
MAKE The Unix/Linux tool make is used to control what needs to be done whenever a source file is updated. This means that the rules about what depends on what are encoded in Makefiles. This sounds complex but in practice is extremely simple using wild card operations whenever possible. For example, each directory on the site has a Makefile which (through a single included template) says that filename.pdf depends on filename.html. When there is an update to an html file in that directory, the pdf file is regenerated automatically. If a file is not touched there is no need to update its pdf file. A master Makefile for the entire site controls how the site is built, simply by listing the set of subdirectories which are included in the site.
ASPELL Spell checking is performed whenever PDF files are generated. This is just a simple invocation of the aspell command at this stage. So, grammatical and other errors are not caught. My aim will be to improve the 'basic' quality of the writing on the site as much as possible, by making use of simple text processing tools like aspell, diction, and style. These will also keep the writing clear (that is my hope at least, please let me know if we are not meeting these goals).
RSYNC Rsync is use to keep the local copy of the site that we work with synchronized with the site on the server. Rsync is a smart synchronization tool. Only files that have changed are transferred to the server. When the site needs to be updated 'make upload' synchronizes the local site with that on the external server.
BASH In some areas of the site (e.g. in creating the list of images which are included in a table linked to the home page) we have used bash scripting to make the process of creating the index as automatic as possible.
NanoBlogger This blog site is created by Nanoblogger and rsync-ed to the main server. Nanoblogger makes use of bash and simple Linux utitlies and leaves the source of the articles easily accessible. If there is ever a need to move to a different blogging tool, then this should be a straightforward migration. However, for now, Nanoblogger is performing just fine.
CVS The content for the site is stored in CVS. This enables me to happily work away with my own local copies and when necessary commit changes to the repository. This sounds grandiose, but in practice is extremely simple. There is no CVS server involved, the CVS repository is simply a set of files on disk.
As is true for all web sites, The Molecular Universe, has grown organically. Pages and technologies have been bolted onto the site from time to time. The openness of the basic construction of the site makes this possible and indeed straightforward. The basic strategy throughout has been the use of Linux (or Cygwin) tools to carry out transformations and control operations, be conscious of the perils of mixing style and content, and avoid the duplication of information.
I hope that this brief overview of The Molecular Universe gives you a sense of how the site is constructed. I know that such information would have been useful when we first started constructing the site. I am sure that few people will want to duplicate the way that The Molecular Universe is constructed exactly. However, having a sense of the tools and thinking behind the site may be useful to you as you think about developing your own site or sites.
Here are two molecules you may have read or heard about:
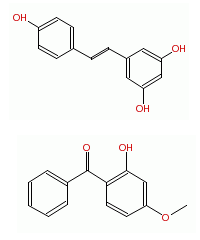
Reseveratrol (which is the upper molecule) is found in red wine, and may be responsible for the so called French paradox. If you haven't come across the French paradox, it is the resilience which French people seem to have to a relatively high fat, unhealthy diet. There is speculation that this is due to the presence of a wonder molecule in red wine, called resveratrol. Of course, nothing has been confirmed, and the explanation may just be that French people eat more healthily and exercise more extensively than researchers appreciate. (And there is always the garlic explanation). Oxybenzone (below) is a molecule found in sunscreen formulations. On an occasional basis claims are made about the potential toxicity of oxybenzone. The FDA is aware of these claims and still ranks oxybenzone as a safe molecule for use in sunscreen.
The chemical formula of both these molecules is: C14H12O3.
However, their three dimensional structures are different (see the image below, again resveratrol is the upper molecule). Different shapes lead to different properties and different properties lead to different effects on living cells.
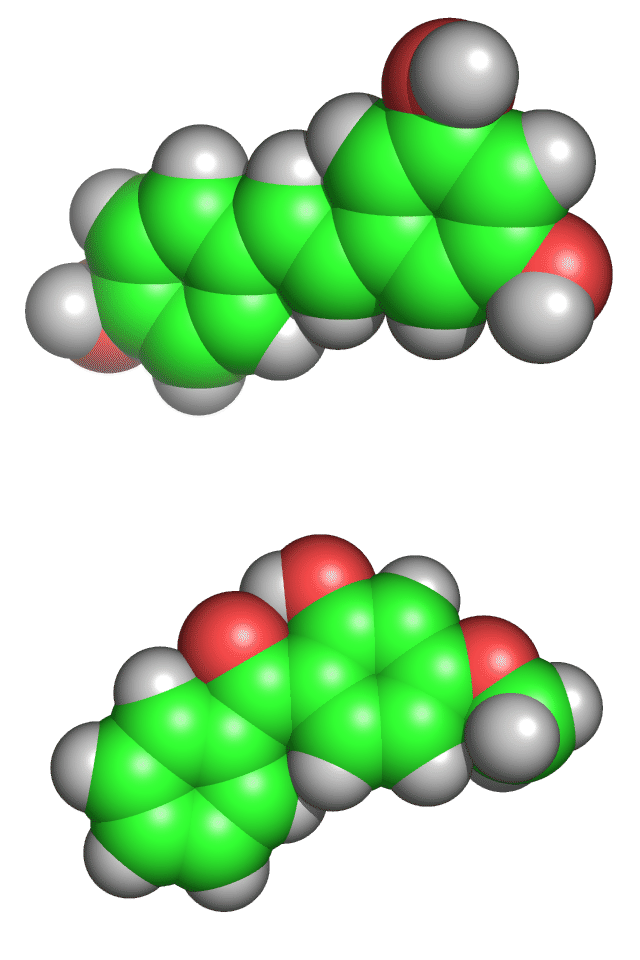
You can be sure that researchers are examining these molecules carefully. When the origin of the resveratrol effect is confirmed, you can then be sure that researchers will be seeking to create improved versions of this molecule which might perhaps improve life expectancies. In the case of oxybenzone, if its toxicity is confirmed, scientist will be seeking modifications which retain the ability to absorb harmful UV radiation yet remove the toxicity.
In 1870 John Tyndall said:
"An intellect the same in kind as our own would, if only sufficiently expanded, be able to follow the whole process from beginning to end. It would see every molecule placed in its position by the specific attractions and repulsions exerted between it and other molecules, the whole process and its consummation being an instance of the play of molecular force."
Computers are now powerful enough that they can track in detail the forces on a system and predict what will become of that system as a function of time. The methodologies are not perfect by any means. Just as in weather forecasting, chemical simulations cannot be run indefinitely, and there are limits on the accuracy that can be achieved. However, in general, computers can simulate many of the properties which might otherwise be measured in the laboratory. Tyndall's 1870 comment has been realized, not by an intellect, but by a set of machines programmed by scientists, based on the laws of nature.
The use of computers in understanding situations is now everywhere. Children, young and old, use computer games to understand sports and driving, tactics and strategy. The military rehearse their plans using war game simulations. Architects allow their customers to walk through virtual buildings. Economists plot out the behavior of the markets on the basis of financial models. All of these activities rely on microcomputers to calculate the possible interactions in the systems and present the important variables be they visual, audio, or numeric to the humans using the system.
Molecular simulations operate in the same way, just as Tyndall foresaw. The computer tracks the attractions and repulsions of the system in minute detail, if necessary tracking the behavior of electrons themselves. The computer then uses this information to put together processes from beginning to end.
Many examples from the worlds of chemistry and materials that have been explored using computers are collected in The Molecular Universe site.
There are a variety of reliable programs for handling chemical systems these days. For example, many chemists use CambridgeSofts's ChemDraw to sketch and rapidly convey chemical information. For chemical information databases there are the products of MDL, now developed and marketed by Symyx. For 3D information and modeling there are a variety of free products, such as PyMol, which allow chemists, biochemists, and crystallographers to explore the shape and structure of important molecules.
Software for chemistry is a field that has been in development for decades. The payoffs in this field are significant, when the number of distinct small molecules that can be synthesized outnumbers the count of atoms in the universe, knowing where to head for particular chemical properties is very valuable. Making the correct molecule can be the difference between life and death, financial success and bankruptcy, and routine drudgery and efficient research and development.
Unsurprisingly then, there have been many attempts to harness computers to manage and predict chemical information. Many of these attempts have led to very successful software products, ChemDraw, for example, has generated a significant profit for its developers for many years.
One product that always impressed, if for nothing else, by the breadth of its ambition, was Polygen's CENTRUM. Here is a link to the CENTRUM product marketing materials of around 20 years ago. CENTRUM was ahead of its time. Before PCs had taken root in the laboratory, CENTRUM was designed to support document based collaboration. In many ways it was a product which anticipated the document sharing and publishing capabilities which have only recently become possible and are still far from commonly used. CENTRUM was designed to be chemically aware. It had a chemical sketching capability, links to academic and industry standard software packages, provided an integrated spell checker, and email capability. For 1987 it was ambitious. (Here is some more of the history of software in chemistry.)
Is there as much ambition in the world of chemical software now? Computers are a little faster than they were in the 1980s (10 MHz versus 2GHz clock speeds, for example). Hard disk drive capacities are larger by orders of magnitude. Prices have dropped by an order of magnitude in real terms. However, the ambition of many of the commercial chemistry software developers seems to have waned. There are pockets of ambition in academia, in distributed protein folding and parallel ligand docking, for example, but in commercial software development currently at least, the focus is on consolidating the ambition of the past.
For those with future ambitions, the extraordinary potential of today's compute resources, storage capabilities, distributed computing technologies, and information sharing infrastructures, present an exciting opportunity for the development of the next generation of chemically aware software products!
I was impressed this morning to see that YouTube carries several movies of molecular systems.
Here is one, for example, which shows a protein folding simulation.
Folding a protein takes computer power and science. If you want to know how such a calculation is accomplished, this article on The Molecular Universe provides details: Computers, Molecules and Materials.
Here is another YouTube movie, this one is a combination interview and animation.
In this movie the interviewee compares protein folding to parallel parking, which is an interesting analogy. Then he goes on to compare the protein to a drunken person trying to parallel park, which is even better! However, the video is a little misleading; the protein does not learn how to pack based on its past attempts, as even a drunken parallel parker would. Instead, it is the lowering of the energy of the system which guides the protein into its native configuration.