Sun Sep 21 17:13:20 PDT 2008
Naphthalene: From Interstellar Medium to Mothballs
Naphthalene is a simple molecule. It contains just ten carbon atoms and eight hydrogen atoms. You can see its structure, both in two dimensional chemist's sketch form and in three dimensional space filling form below. (The hydrogen atoms are not shown in the two dimensional sketch - their positions are infered on the basis of the number of bonds that the carbon atoms possess).
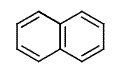
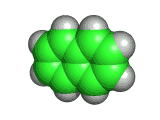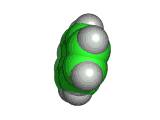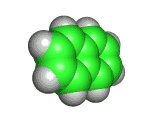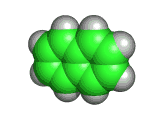
Although naphthalene is simple it is useful. It has long been used to repel moths and other insects, and sold for this purpose in the form of mothballs. And, surprisingly, despite naphthalene's long use, there have been few sensational scares concerning its toxicity to humans. However, naphthalene is not something any human should consume - and in fact eating mothballs or inhaling large amounts of naphthalene will do you no good at all.
Naphthalene was one of the first organic compounds to be examined using x-ray crystallography. William Bragg published the structure of naphthalene in 1921, nine years after his son, Lawrence Bragg, had shown how diffracted x-ray intensities could reveal the atomic structure of crystals. Now x-ray crystallography is used to determine the structure of molecules with thousands of atoms. However, in 1921 the detailed structure of a molecule such as naphthalene was extraordinary. For the first time, chemists could see in great detail the structures of molecules that they were working with in the laboratory. The 1920s also saw developments in quantum mechanics and soon both these branches of science were able to interact. Quantum mechanics provides predictions and explanations for the observations of crystallography.
Naphthalene, despite its simplicity, continues to be of interest. It is useful as an intermediate for the construction of more complex molecules. Naphthalene is found in meteorites and coal. Recently it has been found by astronomers in the interstellar medium, the very diffuse clouds of material and molecules between stars.
So when you smell naphthalene, which is quite recognizable given its use as an insect repellant, remember that it was one of the first organic molecules to have its structure determined by crystallography and that there are vast numbers of naphthalene molecules between the stars!