Sun Nov 9 20:28:24 PST 2008
Zeolites!
Zeolites, like ZSM-11 which is shown below, are important industrially. Zeolites possess pores in their structures which have sizes similar to the size of molecules. Zeolites can also be made to have acidic properties. So, when an acid catalyst is required which can target certain sizes and shapes of reactant and product molecules, zeolites find a range of applications.
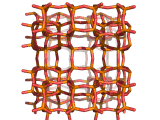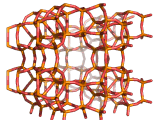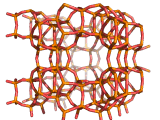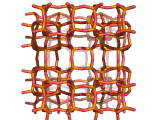
In fact, all of the molecules of gasoline in a typical car have encountered a zeolite catalyst like the one shown above. Oil refineries use zeolite acid catalysts to modify, or crack, crude oil and maximize the output of molecules suitable for use as fuels.
Zeolites are also used in creating specific isomers, avoiding the need for separations following acid catalyzed reaction.
If you look at the image of ZSM-11 you will see why it is special. It contains openings which can admit molecules of certain sizes. If a molecule is too large it will not be able to gain access to the interior of the zeolite, and as the acid sites which the zeolite possesses are in its interior, no acid catalyzed reactions will occur. However, if a molecule is small enough to enter a zeolite it will be rapidly attacked by the protons of the zeolite's acid sites, and interesting chemistry will occur.